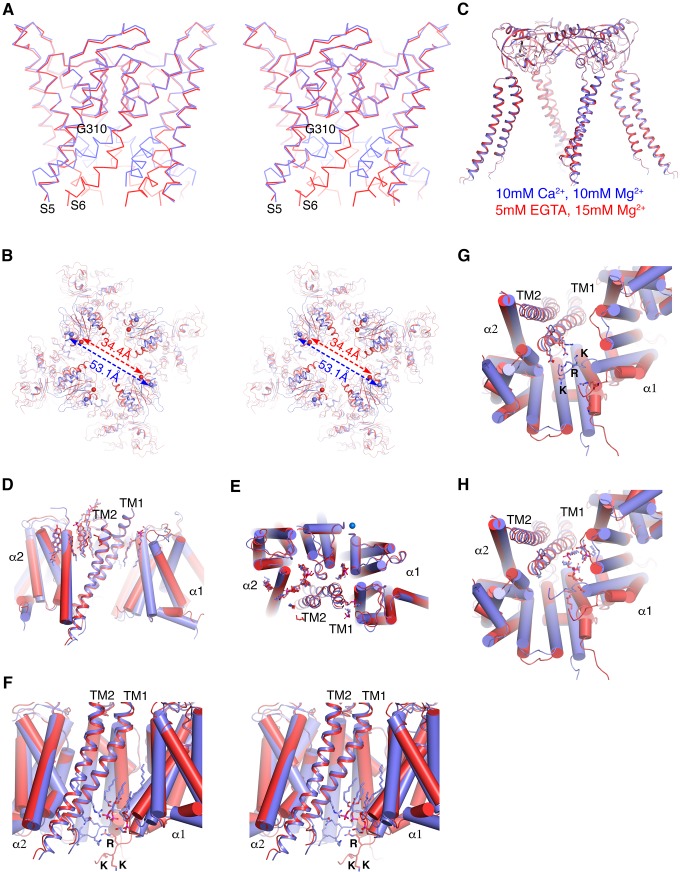
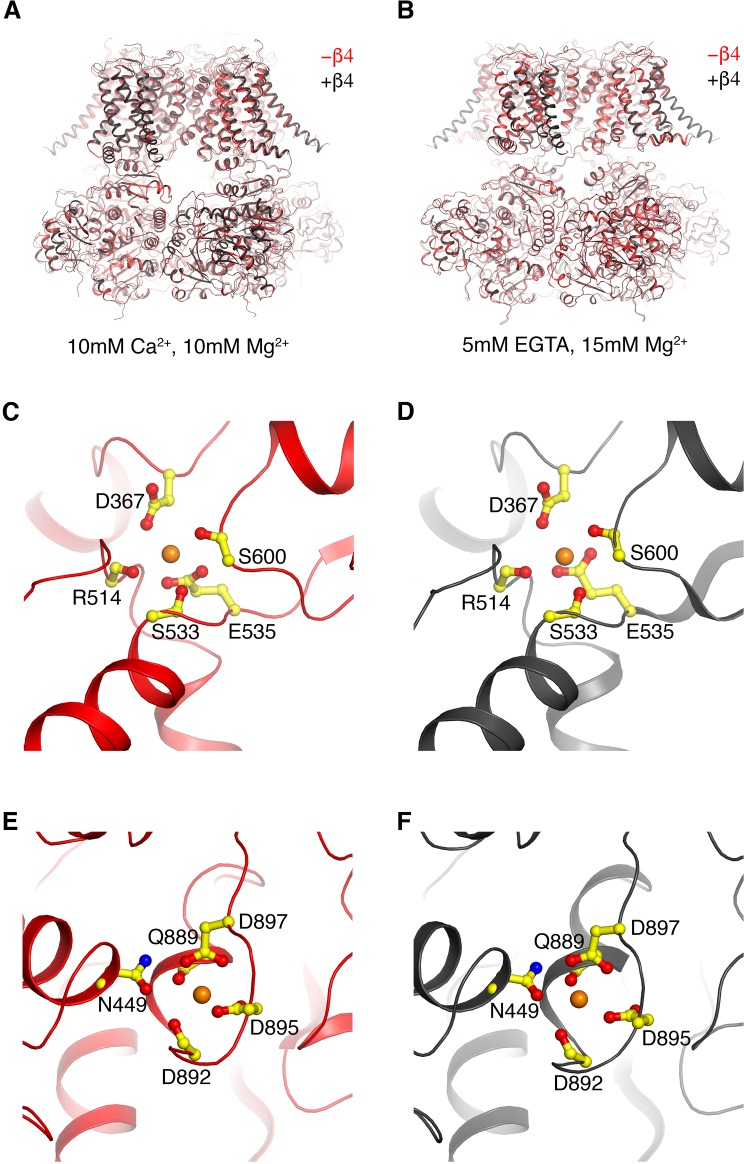
Figure 5. Ca2+gating mechanism of human Slo1 in the absence and presence of β4 subunit.
(A) Ca2+-induced conformational changes in the pore domain of human Slo1-β4 channel complex. Superposition of the pore domain in the absence (red Cα trace) and presence (blue Cα trace) of Ca2+ is shown in stereo. The gating hinge residue G310 on inner helix is labeled. (B) Ca2+-induced conformational changes in the gating ring of human Slo1-β4 channel complex. Superposition of the gating ring (aligning the RCK2 domain) in the absence (red) and presence (blue) of Ca2+ is shown in stereo. The spheres indicate the position of Cα atoms of Gly334 at the beginning of RCK1 domain. Distances between the Cα atoms of Gly334 on opposing RCK1 subunits are labeled. (C) Ca2+ produced essentially no conformational changes in the β4 tetramer. Superposition of the β4 tetramer in the absence (red) and presence (blue) of Ca2+ is shown. (D, E) Ca2+ produced minimal conformational changes in the Slo1-β4 interfaces near the membrane outer leaflet, including the positions of lipid molecules, viewed parallel to membrane (D) or from the extracellular side (E). Superposition of the Slo1-β4 channel complex in the absence (red) and presence (blue) of Ca2+ is shown, aligning the transmembrane domain. For clarity, only one β4 subunit (as ribbons) and the two interacting Slo1 subunits (α1 and α2) (as cylinders) are shown. Lipids at the Slo1 and β4 TM1 outer leaflet interface are shown as sticks. K+ ions in the selectivity filter are show as marine spheres (E). (F) Ca2+-induced conformational changes in the Slo1-β4 interfaces near the membrane inner leaflet. Superposition of the Slo1-β4 channel complex in the absence (red) and presence (blue) of Ca2+ is shown in stereo, viewed parallel to the membrane, aligning the transmembrane domain. Sidechains of the β4 TM1 facing the S6-RCK1 linker as well as the three positively charged residues on S6-RCK1 linker (‘RKK’) are shown as sticks. Lipids at the Slo1 and β4 TM1 inner leaflet interface in the Ca2+-bound state are also shown as sticks. (G) Superposition of the Slo1-β4 channel complex in the absence (red) and presence (blue) of Ca2+ viewed from the intracellular side, aligning the transmembrane domain. Color and representation schemes are the same as in panel (F). Sidechains of the β4 TM1 facing the S6-RCK1 linker as well as the three positively charged residues on S6-RCK1 linker (‘RKK’) are shown as sticks. (H) The same superposition as in panel (G) with lipids at the Slo1 and β4 TM1 inner leaflet interface in the Ca2+-bound state (blue) and Ca2+-free state (red) shown as sticks.