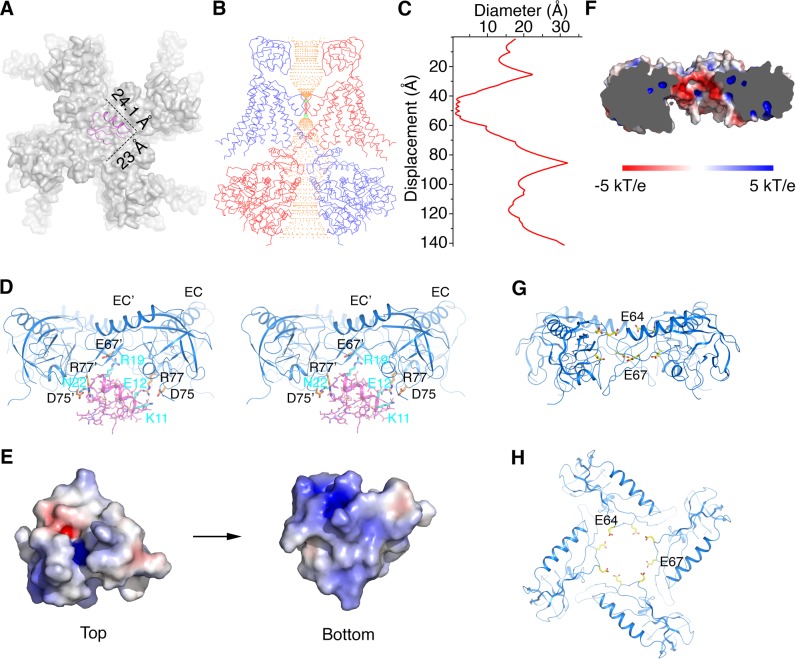
Figure 6. Structural basis for modification of Slo1 toxin sensitivity by β subunits.
(A) CTX docked onto the Slo1 channel based on the crystal structure of the CTX-Kv1.2–2.1 paddle chimera (PDB 4JTA). Only the transmembrane domain of Slo1 is shown (gray surface) and CTX is shown as pink ribbons. Dimensions of CTX in the plane parallel to membrane are indicated. (B) Central conduction pore of the open Slo1-β4 channel complex generated with Hole (Smart et al., 1996). For clarity, only two opposing subunits of Slo1 and β4 are shown (blue and red Cα traces). Pore radius: red,<1.15 Å; green, 1.15 to 2.30 Å; orange,>2.30 Å. (C) Diameter of the central pore. The van der Waals radius is plotted against the distance along the pore axis. (D) The EC domain tetramer of β4 provides potential new toxin binding sites. The EC tetramer is shown as blue ribbons. CTX is shown as pink sticks and ribbons. Potential new CTX binding sites on the EC domain and the corresponding interacting residues on CTX are shown as sticks and colored according to atom type. (E) Electrostatic surface potential of CTX viewed from the extracellular side (‘Top’) or the opposite side (‘Bottom’), calculated with APBS. (F) Negatively charged inner surface of the central pore formed by the EC tetramer of β4 subunits, calculated with APBS. (G, H) Two rings of negatively charged residues E64 and E67 on the EC domain of β4, facing the central pore axis, viewed parallel to the membrane (G) or from the extracellular side (H). EC domains are shown as blue ribbons. Sidechains of E64 and E67 are shown as sticks and colored according to atom type.
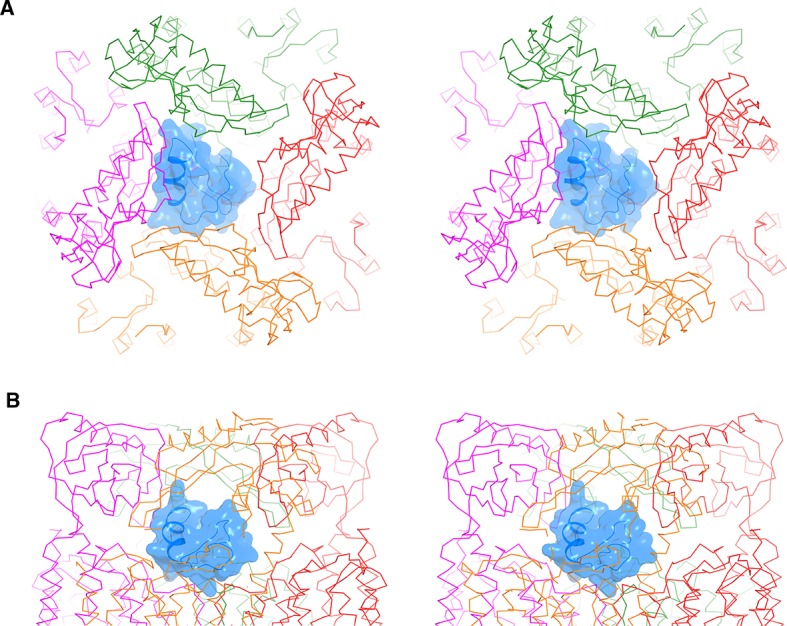
Figure 6—figure supplement 1. CTX docked onto the Ca2+-bound hsSlo1-β4 channel complex.