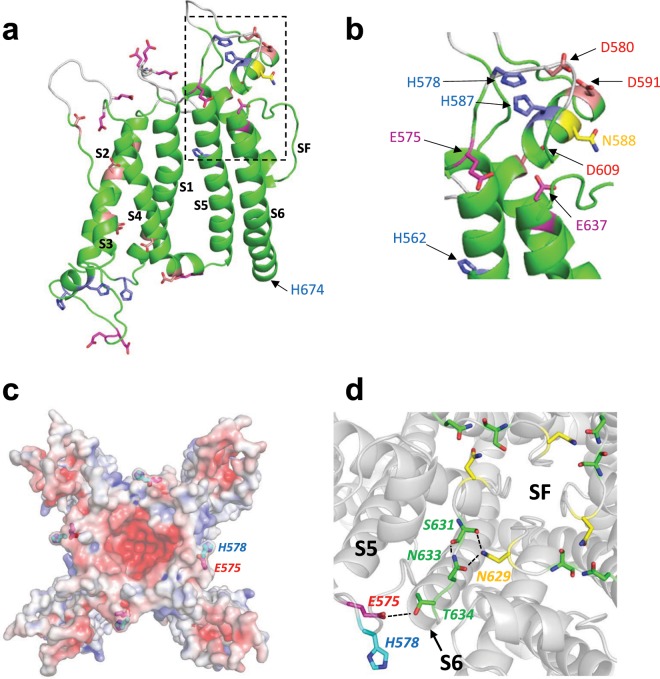
Figure 5.
Structural context of target titratable residues. (a) Single subunit of the cryo-EM structure of the hERG1a channel24 with transmembrane domains labelled. Regions of extracellular loops colored white are absent in the cryoEM structure (PDB:5VA1)24 and were modelled into the structure using Modeller v9.1734 (https://salilab.org/modeller/). The region enclosed in the dashed box comprises most of the extracellular turret between helices S5 and S6, and is expanded in (b). (c) Location of the E575/H578 pair mapped onto the electrostatic surface (at pH 7.4; 0.15 mM NaCl) on the extracellular side of the hERG1a channel. (d) The E575/H578 motif is linked via an E575 (on S5) –T634 (S6) charge/hydrogen bond interaction to a hydrogen-bonded network involving N633, S631 with N629 on adjacent subunits of the hERG1a channel that forms a ring around the top of the selectivity filter (SF).