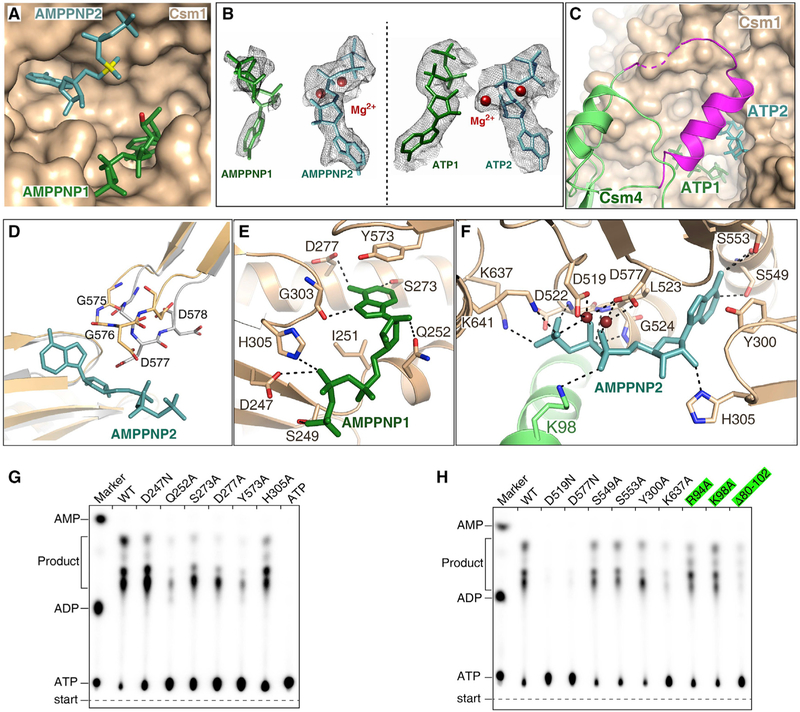
Figure 5. Two ATP or AMPPNP Bind in the Palm Domains of Csm1 Subunit.
(A) Two AMPPNP molecules bind in the pocket formed by two Palm domains. AMPPNP1 (in green) and AMPPNP2 (in teal) bind to Palm1 and Palm2 domains, respectively. The a-phosphate group of AMPPNP2 and the O3′ atom of AMPPNP1 are shown in yellow and red, respectively.
(B) Cryo-EM map for two AMPPNP molecules in the CTR-bound Csm complex (left) and for two ATP in the NTR-bound Csm complex (right). Two Mg2+ are shown as brown spheres.
(C) Csm4 residues 82–104 (in magenta) cover the ATP binding pocket upon ATP binding, whereas they are disordered in the absence of ATP.
(D) Structural comparison the GGDD motif in the CTR-bound Csm complex either in the presence (wheat) or absence (gray) of AMPPNP, showing that the GGDD motif undergoes a conformational change upon the AMPPNP or ATP binding.
(E) Magnified view of the interactions between AMPPNP1 and the Palm1 domain.
(F) Expanded view of the interactions between AMPPNP2 and the Palm2 domain.
(G) Effect on cOAs synthesis of mutations of Csm1 residues that interact with the AMPPNP1.
(H) Effect on cOAs synthesis of the mutations of Csm1 and Csm4 residues that interact with the AMPPNP2. The Csm4 mutants are highlighted in green.
See also Figure S5.