Abstract
Intervertebral disc degeneration associated back pain is the most common cause of disability worldwide; however, no safe and effective treatments have been available. Here, we report a new functionalized nanofullerene conjugated with a peptide that binds specifically to a formyl peptide receptor-1 (FPR-1) expressed on activated macrophages. The new nanoparticle (aka FT-C60) was synthesized by conjugating carboxyl-C60 with the primary amine group of the peptide with a fluorescence dye for easy detection. The new nanoparticle was characterized by X-ray photoelectron spectroscopy, mass spectroscopy, and gel electrophoresis. It possessed effective radical (hydroxyl and superoxide anions) scavenging capabilities in electron paramagnetic resonance spectroscopy. In cultured cells, the nanoparticle FT-C60 demonstrated preferential binding to FPR-1 on activated macrophages and significantly attenuated mRNA expressions of proinflammatory factors including interleukin-6, interleukin-1, tumor necrosis factor-alpha, and cyclooxygenase-2. In vivo animal studies exhibited that a single intravenous injection of FT-C60 effectively alleviated pain in an established mouse model of radiculopathy for up to post-operation day (POD) 12. Ex vivo near-infrared fluorescence imaging of the mouse spine confirmed the targeting property of FT-C60 toward the injured disc on POD 14. Quantitative analysis of histological staining on spine sections showed that nanoparticle FT-C60 dramatically reduced inflammation at the local injury site compared to injury only on POD 7. In summary, we developed a novel targeted nanoparticle for treatment of lumbar radiculopathy by systemic delivery. This is a first-of-its-kind study for developing a novel class of targeted and systemic nanoparticle therapeutics to treat degenerative disc diseases.
Keywords: intervertebral disc degeneration, low back pain, targeted therapy, fullerene, formyl peptide receptor-1
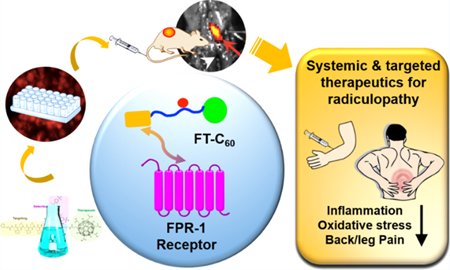
Graphical abstract
1. INTRODUCTION
The most recent Global Burden of Disease reported back pain as the most common cause of disability worldwide.1–3 Back/leg pain is the most common health problem with a prevalence of over 80% and an estimated annual cost of $100 billion in the United States.3,4 Although various factors may contribute to the pathogenesis of back pain, intervertebral disc (IVD) degeneration has been considered as a major cause.5,6 Unfortunately, this devastating condition can only be treated with symptomatic relief interventions, as no effective and disease-modifying medications are yet available.7,8 Nonsteroidal anti-inflammatory drugs (NSAIDs) such as diclofenac, ibuprofen, and naproxen are the most commonly used medications for low back pain. However, it is a well-known fact that NSAIDs can cause stomach ache and bleeding, limiting their usability. Opioid-based analgesics are somewhat effective in alleviating pain symptoms; however, they do not target the pain source, and opioid overuse may cause severe substance abuse and/or drug tolerance. Epidural steroid injection (ESI) serves as a reasonable treatment to reduce inflammation and numb the pain via inhibition of prostaglandins in the arachidonic acid signaling cascade. However, ESI is only effective in some patients and can potentially lead to serious complications such as infection and hematoma. Therefore, it has become an urgent and unmet medical need to develop a new therapeutic strategy to resolve painful radiculopathy, such as a systemic and targeted therapy that is a safe, simple, and effective way.
Despite numerous pathogenic factors contributing to the initiation and progression of degenerative disc diseases, inflammation and the dynamic interplay of immune cells play critical roles in disc degeneration and associated low back/leg pain.6,9 IVD is the largest avascular organ in the human body and considered as an immune-privileged site. A normal disc is an immune-privileged organ, whereas a herniated disc evokes complex immune responses. The central nucleus pulpous (NP) is particularly isolated from our immune system due to its location between two cartilaginous endplates and inside the dense collagen fibrous structure of the annulus fibrosus (AF).10 In addition to such physiological barriers, disc cells actively resist the invasion of immune cells shown by the expression of Fas ligand (FasL), a characteristic of the immune-privileged site. During disc herniation, NP tissue extrudes into epidural space, evoking autoimmune responses, abundant infiltration of immune cells (e.g., macrophages and dendritic cells, T and B cells, etc.), and cytokine production by both disc and immune cells exacerbating the inflammation and pain.5 Approximately 66% of surgically obtained herniated human disc specimens show abundant macrophage infiltration, which suggests that they are a prominent source of inflammation and pain.11–13 Our previous study also illustrated abundant macrophage infiltration near the site of disc hernia in a mouse model of disc herniation.14,15 All these findings support that activated inflammatory cells such as macrophages at local disc hernia sites might be a valid target for therapeutic development.
Formyl peptide receptor-1 (FPR-1) is highly expressed in leukocytes such as neutrophils, monocytes, and macrophages under an inflammatory insult. It has been extensively reported that circulating monocytes transform into macrophages during the late phase of inflammatory diseases for phagocytic cleanup. In our previous study, we demonstrated that the intravenously administered cFlFlF peptide specifically bound to FPR-1 expressed on activated macrophages/monocytes at the local disc hernia site,14 empowering targeted drug delivery to disc hernia sites—the source of pain. Meanwhile, nanoparticle fullerene (C60–C80) is composed of 60–80 carbons in hollow sphere form that is ~1 nm in diameter. Fullerene and its derivatives have showed promising therapeutic potential in a range of pathological conditions involving oxidative stress and inflammation.16–18 Bioactivity examples of fullerene may include antimicrobial activity, neuroprotection, DNA cleavage, apoptosis, ion channel inhibition, and inhibition of amyloid formation.19,20 Due to the high degree of unsaturation, the molecule is able to effectively trap free radicals as a radical sponge. Fullerenes are intrinsically superior to growth factors, cytokines, and enzymes because of their long-lasting activity and cell membrane-penetrating ability. We are the first to discover the essential role of nanomedicine in treating degenerative disc diseases. We demonstrated that fullerol (water-soluble form of fullerene) possessed pleiotropic therapeutic functions in the complex pathological context of discogenic back/leg pain by harmonizing pathological cross-talks among the disc, spinal nerve, and immune system via neuroprotective, disc regenerative, anti-inflammatory, and analgesic effects.17,20,21
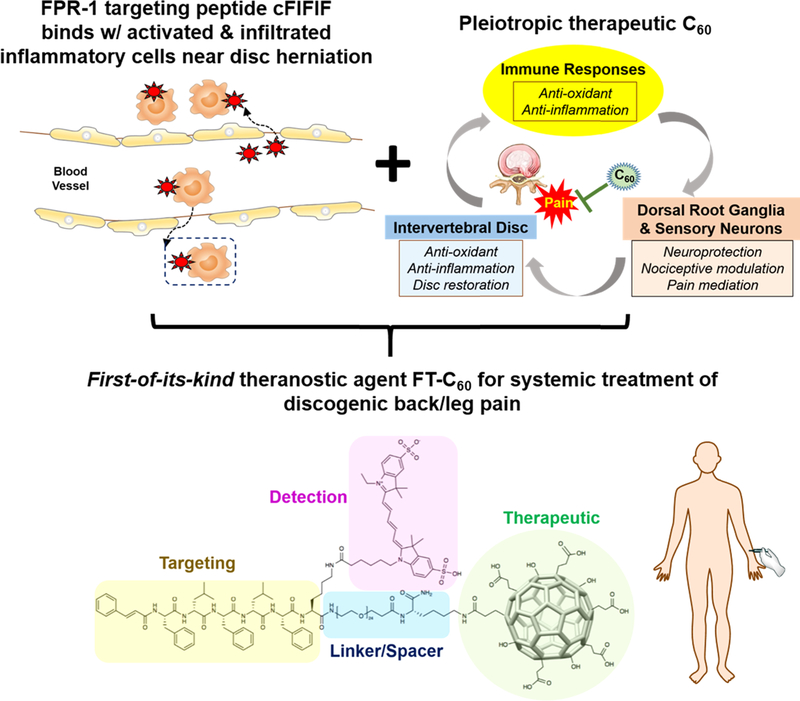
As shown in Figure 1, we aim to develop a new FPR-1 targeted C60 nanoparticle (referred as FT-C60) as a promising nanomedicine to alleviate lumbar radiculopathy by intravenous administration. Our FT-C60 has a modular design, including functionalized C60 as the therapeutic moiety, polyethylene glycol (PEG) and lysine (K) as the linker and spacer, and the cFlFlF peptide as the targeting modality, and a near-infrared fluorescence dye Cyanine 5 (Cy5) to ease detection. This is a first-of-its-kind study for developing a novel class of targeted nanoparticles to treat discogenic pain by systemic delivery and demonstrating its potent anti-inflammatory and pain alleviation effect in a mouse model of lumbar radiculopathy.
Figure 1.
Schematic illustration on therapeutic and targeting mechanisms of the newly developed first-of-its-kind theranostic nanoparticle FT-C60, empowering systemic delivery of nanomedicine to treat discogenic back/leg pain.
2. RESULTS AND DISCUSSION
2.1. Chemical Synthesis and Characterization of Targeting Peptide, Functionalized C60, and FT-C60 Conjugate.
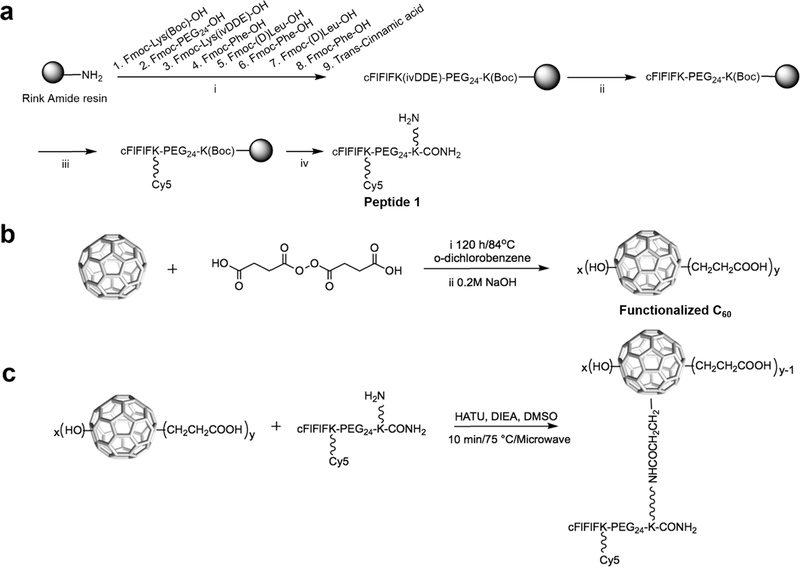
Chemical synthesis of fluorescence dye Cy5 labeled peptide sequence [cFlFlFK-PEG24-(Cy5)-K] was performed using established solid-phase peptide chemistry as shown in Figure 2a and was characterized by MALDI-TOF-MS with detected molecular weights of 2837.44 [M–H], 2838.354 [M], and 2839.358 [M+H] (Figure S1), which was also confirmed with LC-ESI-MS (Figure S2). In our initial design, two peptide structures (peptide 1 in Figure 2a and peptide 2 in Scheme S1) were designed and synthesized with their reactive primary amine at two different positions, respectively.
Figure 2.
Synthesis of (a) targeting moiety peptide 1 [cFIFIFK(Cy5)PEG24K(NH2)CONH2], (b) functionalized C60 and (c) FT-C60 conjugate. Reagents and conditions: f (a), (i) standard solid-phase Fmoc chemistry; (ii) 2% NH2NH2; (iii) Cy5, DIC, HOBt; (iv) 95% trifluoroacetic acid (TFA); (b), (i) o-dichlorobenzene, 120 h/84 °C (ii) NaOH (50 mL, 0.2 M); (c),HATU (0.26 μmol), DIEA (4.32 μmol), DMSO, 10 min/75 °C, and microwave (CEM liberty 1 microwave system). The resulting solution was purified by polyacrylamide desalting columns and RP-HPLC.
Functionalized C60 was prepared as in Figure 2b with in situ prepared starting material succinic acid acyl peroxide (Scheme S2). According to XPS data, the empirical chemical formula of functionalized C60 was calculated as C60(OH)21(CH2CH2COOH)13 with an estimated mass of 2026 (Figure S3). The MALDI-TOF mass spectra suggested that the molecular weight of functionalized C60 (Figure S4) was 2387, corroborating the formula C60(OH)28(CH2CH2COOH)16 (mass estimated:2364) with a Na+ ([M + Na])+.
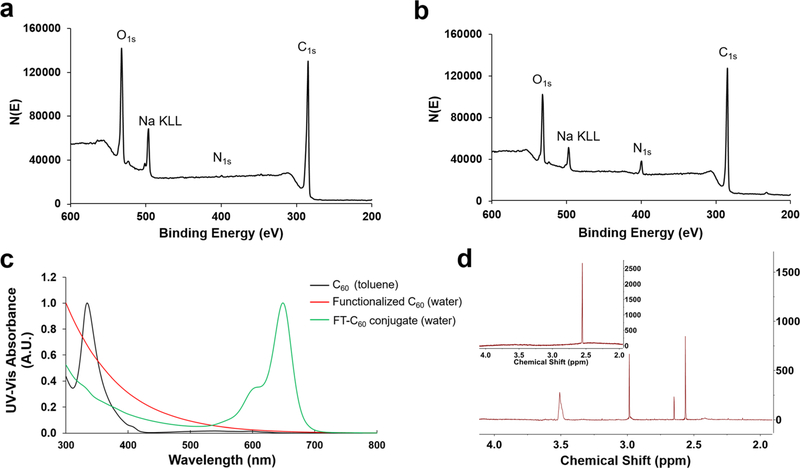
Chemical conjugation of peptide and functionalized C60 was attempted in various approaches, including DIC/HOBt, EDC/NHS, and microwave-assisted DIEA/HATU coupling. Conjugation of FT-C60 was successfully achieved with peptide 1 and carboxyl-C60 using microwave-assisted DIEA/HATU coupling method (Figure 2c). Functionalized C60 and FT-C60 were characterized by various techniques, such as UV-vis spectroscopy, mass spectroscopy, XPS, NMR, and gel electrophoresis. The conjugation of peptide 1 and functionalized C60 was confirmed by MALDI-TOF-MS (Figures S4 and S5), XPS survey spectra (Figure 3a,b), UV–vis (Figure 3c), 1H NMR (Figure 3d), and native polyacrylamide gel electrophoresis (Figure S6). It is well recognized that peptides and other functional groups are readily cleaved from the C60 structure during ionization by MALDI-TOF-MS. The molecular weight of newly synthesized conjugate FT-C60 was estimated to be ~5200, which possibly suggested a 1:1 ratio of peptide-to-C60 conjugation by MALDI-TOF-MS (see the Supporting Information). As shown in the survey spectra of the functionalized C60 and FT-C60 conjugate in Figure 3a,b, the peaks centered at binding energies of 284.4, 400.4, 497.4, and 533.4 eV represent C1s, N1s, Na Auger, and O1s, respectively. The appearance of the peak intensity for N1s for the FT-C60 conjugate was readily observed after chemical conjugation in comparison with the functionalized C60 alone. This clearly suggests the successful conjugation of the peptide in the final FT-C60 conjugate. Based on the peak intensities of N1s and C1s, the XPS data suggested that the peptide-to-functionalized C60 ratio was estimated to be 1.6:1, which was consistent with a similar value from the 1H NMR data vide infra. In addition, the UV–vis absorbance spectrum also revealed that the FT-C60 conjugate exhibited the characteristics of both the peptide and functionalized C60 with a unique absorbance centered at 650 nm, indicating the presence of Cy5 and substantiating the successful conjugation (Figure 3c). As shown in Figure 3d, the 1H NMR spectra of both FT-C60 conjugate and functionalized C60 exhibited the methylene hydrogens that were adjacent to the carboxyl group on the C60 cage at ~2.55 ppm. In addition, the 1H NMR spectrum of the FT-C60 conjugate also exhibited a broad PEG 1H peak centered at 3.5 ppm, which again suggested the successful conjugation of the peptide to the fullerene C60 cage. Integration of the PEG versus methylene hydrogens in the 1H NMR spectrum suggested a peptide-to-C60 ratio of 1.5:1, consistent with the XPS data vide supra (see the Supporting Information for detailed 1H NMR data).
Figure 3.
Characterization of FT-C60 conjugate. XPS survey spectra of (a) functionalized C60 and (b) FT-C60 conjugate. The peak centered at a binding energy value of 400.4 eV represents N1s. The peak intensity of increased significantly after conjugating the peptide to the functionalized C60, indicating the success of the conjugation process. The peaks centered at binding energies of 284.4, 497.4, and 533.4 eV represent C1s, Na Auger peak, and O1s, respectively. (c) UV-vis absorbance spectra of C60, functionalized C60, and the FT-C60 conjugate in toluene and water. The C60(OH)28(CH2CH2COOH)16 lost the characteristic peaks for C60, indicating that the introduction of the functional groups changed the electronic structure. The peak in the range of 600 to 700 nm was characteristic of the Cy5 on the peptide, which suggested the successful conjugation of the peptide onto the functionalized C60. Blue: C60 in toluene. Red: C60(OH)28(CH2CH2COOH)16 in water. Green: the conjugate in water. (d) 1H NMR of the FT-C60 conjugate and functionalized C60 (inset) in D2O. The methylene protons in the functionalized C60 were shown as a singlet at 2.55 ppm. The same methylene protons contributed by the functionalized C60 and a PEG proton peak at 3.5 ppm were detected in the 1H NMR spectrum of the FT-C60 conjugate, further confirming the conjugation.
Since hydrophilicity/ hydrophobicity plays a critical role for biocompatibility and PK/PD profiles, the partition coefficient (log P) of the conjugate FT-C60 was assessed. After conjugation, the hydrophilicity of FT-C60 (log P, −1.25 ± 0.13) increased compared to the targeting moiety peptide 1 (log P, −0.84 ± 0.13), which was suitable for downstream biological applications (Figure S7). It is noteworthy that the log P value was directly affected by structural modification, which would in turn affect the bioavailability and downstream biological evaluations.
2.2. Newly Synthesized FT-C60 Possessed Strong Radical Scavenging Capability.
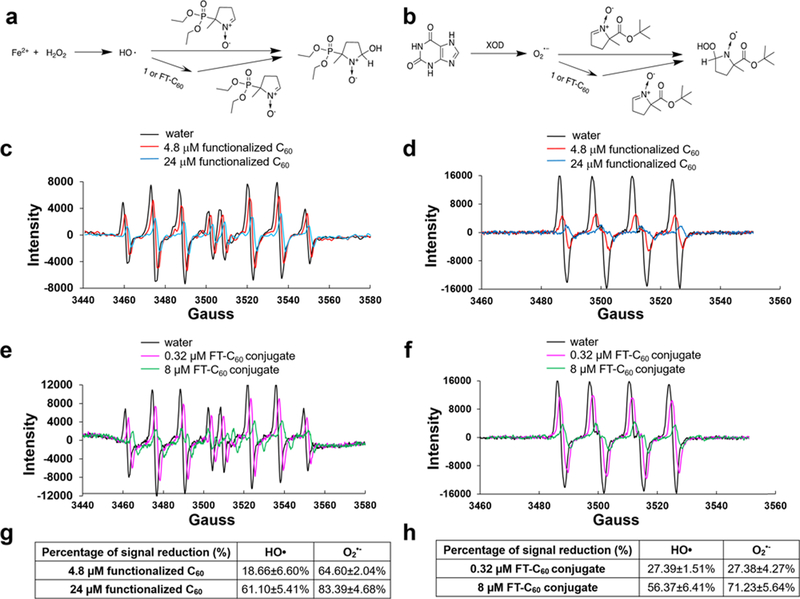
The electron paramagnetic resonance (EPR) technique was used to evaluate the scavenging capability of the functionalized C60 and the conjugate FT-C60 to eliminate ˙OH and O2− in a cell free system.17 Both radicals are among the most common reactive oxygen species (ROS) in the biological system with profound effects in inducing oxidative stress and proinflammatory responses. As shown in Figure 4a, the EPR assay was based on the competition between 5-diethoxyphosphoryl-5-methyl-1-pyrroline N-oxide (DEPMPO) and the functionalized carboxyl-C60 or the conjugate FT-C60 for ˙OH. The classical Fenton reaction was used to generate hydroxyl radicals, and a spin trapping agent DEPMPO was used to form a stable adduct. The EPR spectrum was acquired, which presented as the signal baseline. As the functionalized C60 (Figure 4c) or the conjugate FT-C60 (Figure 4e) was added, the peak intensity decreased dramatically in a dose-dependent manner, indicating effective hydroxyl radical scavenging. Similarly, Figure 4b illustrated the chemistry mechanism of superoxide radical scavenging by 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO), and both functionalized C60 (Figure 4d) and the conjugate FT-C60 (Figure 4f) could effectively scavenge the O2˙− radical. Figure 4g illustrated the quantitative data of radical scavenging of functionalized C60 and FT-C60 on ˙OH and O2−˙. These data indicated that both the functionalized C60 and the FT-C60 possessed promising antioxidative properties due to their superb radical scavenging capabilities. It was of note that functionalized C60 showed ~61 and ~83% signal reduction for ˙OH and O2−˙, respectively, at a concentration of 24 μM, while conjugate FT-C60 at only 8 decreased ~56 and 71% of the EPR signal (approximately 3 times stronger scavenging capability than that of functionalized C60). This increasing antioxidative potential in FT-C60 might be contributed by the addition of peptide 1 and increased n bonds on the peptide structure, such as the Cy5 moiety, which stabilized the electrons.
Figure 4.
Both functionalized C60 and FT-C60 conjugate showed robust radical scavenging properties detected by EPR. (a) Mechanism of the hydroxyl radical production by the Fenton reaction and captured by DEPMPO. (b) Mechanism of superoxide radical production by xanthine/xanthine oxidase (XOD) system and captured by BMPO. EPR spectra of the hydroxyl radical captured by DEPMPO with and without (c) functionalized C60 and (e) FT-C60 conjugate. EPR spectra of superoxide radicals captured by BMPO with and without (d) functionalized C6o and (f) FT-C60 conjugate. Ultrapure distilled water was used as a control. Table summary of scavenging capabilities of hydroxyl radical and superoxide radical anion by (g) functionalized C60 and (h) FT-C60 conjugate. EPR signal reduction depicted a stronger radical scavenging capability of conjugate FT-C60 compared to functionalized C60 in a dose-dependent manner.
2.3. FT-C60 Preferentially Bound toward LPS Stimulated Macrophages in Vitro.
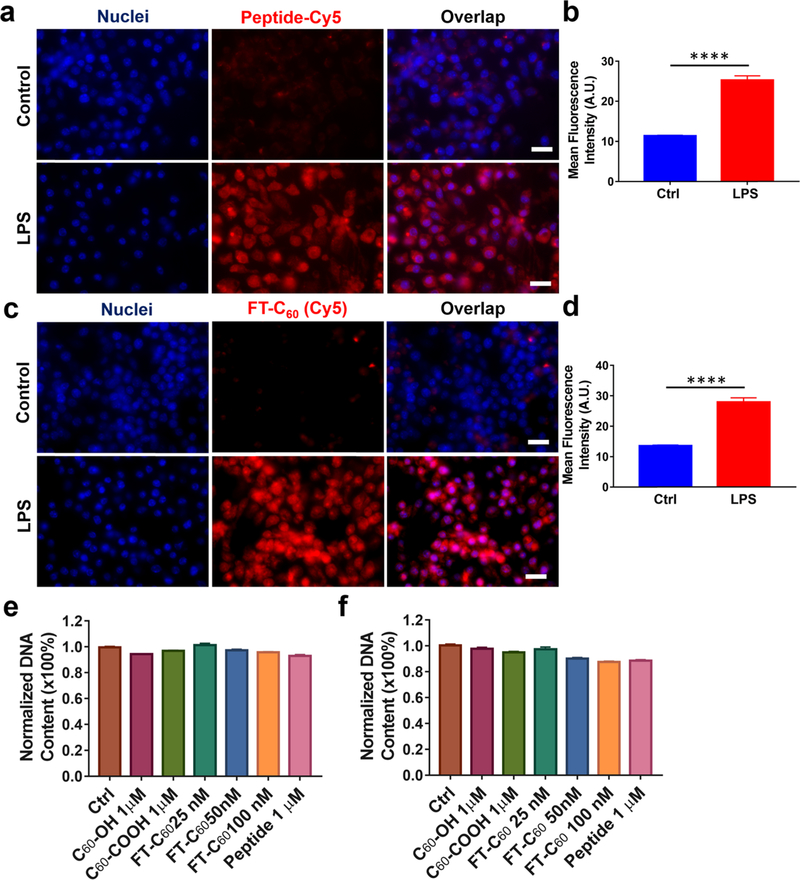
Macrophages are an integral part of the innate immune system to defend the host against pathogens via their phagocytic ability and play critical contributions to the effector phase of the adaptive immune response. They also play pivotal roles in the initiation and progression of degenerative disc diseases, as abundant macrophages were detected in degenerated discs. FPR-1 is a G protein coupled receptor highly expressed in the phagocytic leukocytes. We have designed a synthetic peptide 1 that specifically binds to the FPR-1 receptor on activated macrophages/monocytes during inflammation. Conjugate FT-C60 was designed to carry both an FPR-1 targeting moiety and a therapeutic module—an anti-inflammatory and antioxidative nanoparticle C60. To evaluate whether the conjugate FT-C60 possessed a comparable binding property toward the FPR-1 receptor in macrophages to the peptide itself, we evaluated in vitro binding using a Raw 264.7 macrophage cell line. The Raw 264.7 cell line is a classic cell model to test anti-inflammatory and antioxidative therapeutics in which we have demonstrated abundant induced FPR-1 expression after LPS stimulation.22 As shown in Figure 5a,b, the targeting peptide 1 showed a higher fluorescence intensity (~2.5 times) in LPS-stimulated cells compared to non-LPS treated control (****p < 0.0001). Similarly, newly developed FT-C60 also demonstrated preferential binding toward LPS stimulated macrophages (Figure 5c,d). As expected, these results corroborated with elevated FPR-1 expression in activated macrophages via immunofluorescence staining (Figure S8). The FPR-1 binding specificity of peptide 1 with a larger PEG34k was previously confirmed in peritoneal macrophages from wild type and FPR-1−/− mouse.14 Since FT-C60 exhibited much stronger Cy5 fluorescence than its targeting peptide alone, we adopted a lower dose of FT-C60 (100 nM) in the cellular binding study compared to the peptide alone (1 μM). This data suggested that, after chemical conjugation, the nanoconjugate possessed comparable preferential binding capability to LPS-stimulated FPR-1 expressing macrophages, which laid a solid ground for further evaluation on in vivo targeting.
Figure 5.
FT-C60 showed preferential binding toward LPS stimulated macrophages with negligible cytotoxicity in vitro. Representative fluorescence images suggested both peptide 1 at 1μM (a) and FT-C60 at 100 nM (c) demonstrated preferential binding properties to FPR-1 on LPS-stimulated macrophages compared to non-LPS treated controls. Quantitative analysis of mean cellular fluorescence intensities of peptide 1 (b) and FT-C60 (d) groups confirmed binding to activated macrophages over nontreated control cells (****p < 0.0001, t test). In addition, FT-C60 exhibited negligible cytotoxicity in macrophages after in vitro culture for 1 day (e) and 3 days (f). Note that, for panels (a) and (c), the scale bar was 20 μm. Images were taken at a magnification of 600× using Cy5 and DAPI channels. For panels (b) and (d), 50 cells were analyzed per group.
2.4. FT-C60 Exhibited Negligible Cytotoxicity in Macrophages in Vitro.
Similar to other nanoparticles, physicochemical properties of fullerene and its derivatives are likely to impact their toxicity, including chemical structures, surface modifications, preparation procedures, and exposure doses. In general, nanomaterials that retain >80% cell viability are considered safe for use in biological applications.23,24 Acute and relative long-term toxicities of the nanoparticles were determined by the DNA assay as an approach to assess the cell viability after treatment of a range of concentrations of nanoconjugates. As shown in Figure 5e,f, DNA contents remained the same between control and FT-C60 treated macrophages after 1 day and 3 days of culture at doses of 25, 50, and 100 nM. We further confirmed that commercial fullerol C60-OH, functionalized C60-COOH, and targeting moiety peptide 1 at 1 μM concentration had no cytotoxicity, corroborating with previous studies by others and us.15,17,19–21 Bright-field microscopic images of macrophages subjected to various treatments illustrated a negligible impact on cell morphology and proliferation, as well as the DNA assay shown in the Supporting Information (Figure S9).
2.5. FT-C60 Effectively Inhibited LPS-Induced Inflammation in Macrophages in Vitro.
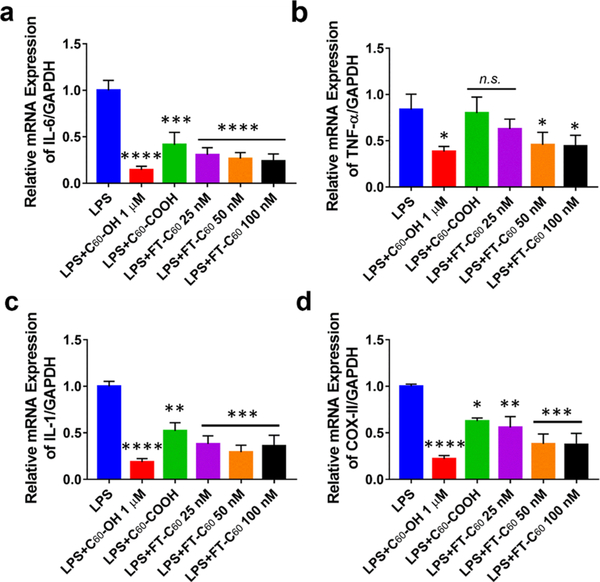
Inflammation plays a crucial role in pain development of intervertebral disc degeneration.25 During inflammation, expression and over-production of proinflammatory factors, such as interleukin (IL)-1, IL-6, tumor necrosis factor-α (TNF-α), and prostaglandin E2 (PGE2), are crucial for triggering progressive activation of immune cells,26 which have been significantly correlated with the symptoms of herniated disc disease.13,27 In Raw 264.7 macrophages, LPS induced proinflammatory gene expression in a time-dependent fashion (Figure S10). As shown in Figure 6, FT-C60 significantly attenuated LPS-induced mRNA expression of cytokines, including IL-6 (Figure 6a), TNF-α (Figure 6b), IL-1 (Figure 6c), and COX-II (Figure 6d). Compared to LPS-stimulated cells, FT-C60 demonstrated a dose-dependent protective effect, which was comparable to well-established fullerol (C60-OH) group (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs LPS treated groups) and functionalized C60. These data demonstrated the promising anti-inflammatory effect of the new conjugate FT-C60 in vitro.28–30 Both housekeeping gene GAPDH and ribosome 18S were used to normalize expression of genes of interest and yielded similar results (Figure S11). The targeting moiety peptide 1 alone did not possess any biological effects under our current experimental conditions (Figure S12).
Figure 6.
FT-C60 effectively inhibited LPS-induced mRNA expression of proinflammatory cytokines in cultured macrophages. FT-C60 suppressed the mRNA expressions of (a) IL-6, (b) TNF-α, (c) IL-1, and (d) COX-II elicited by LPS. These protective, anti-inflammatory effects were also seen in fullerol (C60-OH) and functionalized fullerene (C60-COOH). Raw 264.7 macrophages were treated with LPS (100 ng/mL) overnight. GAPDH was used as a housekeeping gene to normalize the gene of interest. One-way ANOVA with multiple comparisons was used for data analyses. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs LPS treated groups. n.s. indicated not significant different between two groups.
2.6. FT-C60 Effectively Alleviated Radicular Pain via Systemic Targeted Delivery in Mice.
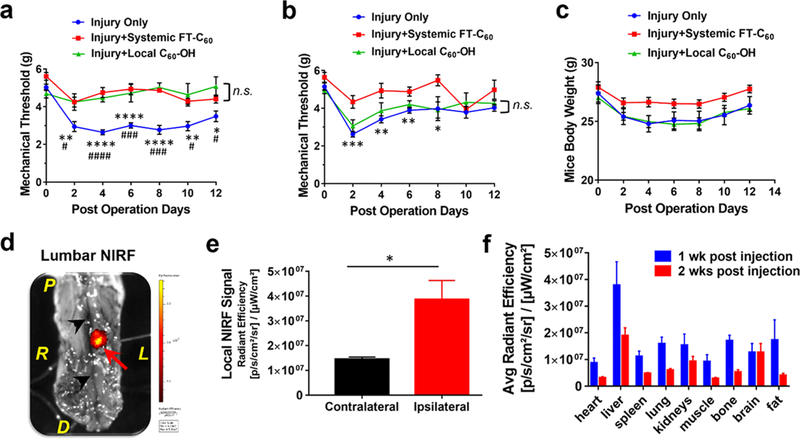
We have previously established an animal model of mouse lumbar radiculopathy secondary to needle puncture induced disc herniation. 15,31 To better simulate the radiculopathy that occurred in human, we exposed and punctured the L4/5 lumbar disc from the far-lateral left side ensuring herniated nucleus protrusion toward the nearby exposed nerve root. An abundance of inflammatory cells including macrophages is infiltrated to the disc herniation/nerve root site, initiating a cascade of proinflammatory responses and evoking pain. The von Frey test, developed by the physiologist Maximilian von Frey, is a method of evaluating mechanical allodynia (painful sensation caused by innocuous stimuli like light touch) in mice and rats and a well-established and commonly adopted method for determining mechanical thresholds in mice. To test the analgesic effect of FT-C60, we administered FT-C60 into the mice via tail vein after surgery and evaluated pain sensitivity via a von Frey filament-based assay every other day post-surgery.32 It was very intriguing that a single intravenous injection of FT-C60 (10 nmol per 20 g mice) effectively diminished ipsilateral mechanical hyperalgesia (pain sensation, lower withdrawal threshold suggests higher pain level) for up to POD 12, similar to intraoperative local administration of fullerol (Figure 7a). For the contralateral mechanical threshold, no significant difference was observed between systemic FT-C60 and local C60 application (Figure 7b). Continuous monitoring of animal body weights revealed that both local C60 administered and injury only groups demonstrated a slight decline of weight (POD 0–8) and then an upward recovery tendency, whereas the FT-C60 treated group maintained a relatively stable trend over the experimental period (Figure 7c). In addition, we clearly visualized much stronger hind leg strength and faster movement in the FT-C60 treated group compared to the injury only group on POD 6 (Video S1). Ex vivo near-infrared fluorescence imaging (NIRF) of the mouse spine clearly depicted focal accumulation of FT-C60 toward inflammatory infiltration sites of the injured disc on POD 7 and POD 14. As shown in Figure 7d and Figure S13, a strong fluorescence signal was clearly discernible on the anterior ipsilateral (left, red arrow indicated) site where the disc was punctured. With the assistance of visualization using a surgical microscope, semiquantitative analysis of ex vivo NIRF imaging confirmed targeted FT-C60 accumulation near the ipsilateral puncture site (~3-fold), compared to the contralateral side of the punctured disc (*p < 0.05) (Figure 7e). These results corroborated with our prior study that a similar FPR-1 targeting peptide (with the same sequence of amino acid but different length and position of PEG) possessed preferential binding to abundant inflammatory cells, such as macrophages around the local disc herniation site in mice.14 With small animal NIRF and single photon emission computed tomography (SPECT) imaging, we also demonstrated specificity of cFlFlF binding to the FPR-1 receptor in detecting local infiltrates at disc hernia using FPR1−/− mice.14 The semiquantitative organ distribution profile illustrated dynamic hepatobiliary clearance of FT-C60 after intravenous administration for 1 week and 2 weeks using IVIS fluorescence imaging, grounding for further pharmacodynamic/kinetic explorations (Figure 7f, Figure S14). NIRF fluorescence signal intensities were presented as average radiant efficiency ([p/s/cm2/sr]/[μW/cm2]). These data suggested that systemic delivery of FT-C60, even at a single administration, could effectively alleviate pain in our animal model of lumbar radiculopathy up to 12 days post injection. Such a long-lasting therapeutic effect of FT-C60 might be attributed to its targeted accumulation to the disc-of-interest site and relatively long half-life in circulation, which appeared as a superior property of FT-C60 to other medications due to the chronic nature of degenerative disc diseases and back pain.
Figure 7.
Systemic delivery of newly developed targeted nanoparticle (FT-C60) effectively attenuated pain in a mouse model of lumbar radiculopathy secondary to disc herniation. (a) Single injection (iv) of FT-C60 (10 nmol per 20 g mice) effectively attenuated mechanical hyperalgesia in a mouse model of lumbar radiculopathy up to POD 12, similar to intraoperative local administration of fullerol C60 (1 μM, 10 μL). (b) Neither injury nor injury + treatment (systemic and local) groups showed significantly altered contralateral mechanical threshold. (c) Animal body weights of injury groups w/or w/o fullerol C60 treatment showed a slight decline first (POD 0–8) and then an upward recovery, whereas the FT-C60 treated group maintained a relatively stable trend over time. (d) Ex vivo near infrared fluorescence imaging (NIRF) of the mouse spine depicted the targeting property of FT-C60 toward inflammatory cells of injured discs on POD 7 (7 days after tail vein injection as well), evidenced by signal registration with the microscopically confirmed disc puncture site (red arrow). P, proximal; D, distal; L, left (ipsilateral); R, right (contralateral); Black arrowheads, midplane of spine. (e) Semiquantitative analysis of the localized NIRF signal supported the significantly higher (~3-fold) NIRF signal (targeted FT-C60 accumulation) near the ipsilateral puncture site, compared to the contralateral side (*p < 0.05). (f) Semiquantitative organ distribution profile of FT-C60 after intravenous administration for 1 week and 2 weeks using IVIS fluorescence imaging illustrated dynamic hepatobiliary clearance of systemic delivered FT-C60. Note: One-way ANOVA with multiple comparisons and multiple t tests were used in panels (a) and (b). For injury only group, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs Injury + Systemic FT-C60, and #p < 0.05, #p < 0.01, < 0.001, ####p < 0.0001 vs Injury + Local C60 group. n.s. indicated not significant between two groups.
2.7. FT-C60 Potentially Reduced Tissue Volume of Inflammatory Infiltration.
We further conducted the histological analysis to assess whether FT-C60 possessed an anti-inflammatory property at the tissue level in our animal model. As shown in Figure 8a,b, massive cell infiltration was observed in the left lateral tissue near the nerve root around the needle puncture trajectory. In addition, a typical chondrogenic change at the disc hernia site was detected in the injured group along with abundant inflammatory infiltration in the surrounding tissue (Figure S15). Customized quantitative histology analysis (Figure S16) was employed on axial spine sections where both degenerative discs and massive inflammatory cell infiltration were present in the injury side near the exposed nerve root. The results affirmed that systemic FT-C60 dramatically reduced calculated inflammatory tissue volume versus the injury only group on POD 7 (Figure 8c,d). As shown in Figure 8e, safranin-O staining showed typical disc degeneration phenotype with loss of NP cells, disoriented annulus fibrosus (AF), and needle puncture trajectory in injured discs of both groups. It was still not clear whether FT-C60 promoted disc regeneration since our focus of this study was to test the effect of pain relief in acute disc herniation. Although the histological analysis presented here was semiquantitative, it demonstrated a promising anti-inflammatory potential of systemic administered FT-C60, corroborating with aforementioned in vitro data and our prior studies.15,20,31,33 In addition, we did not observe any toxicity based upon gross animal behaviors within our in vivo experimental setting. More systemic toxicity studies shall be in sought of when further translation investigation is on the horizon.
Figure 8.
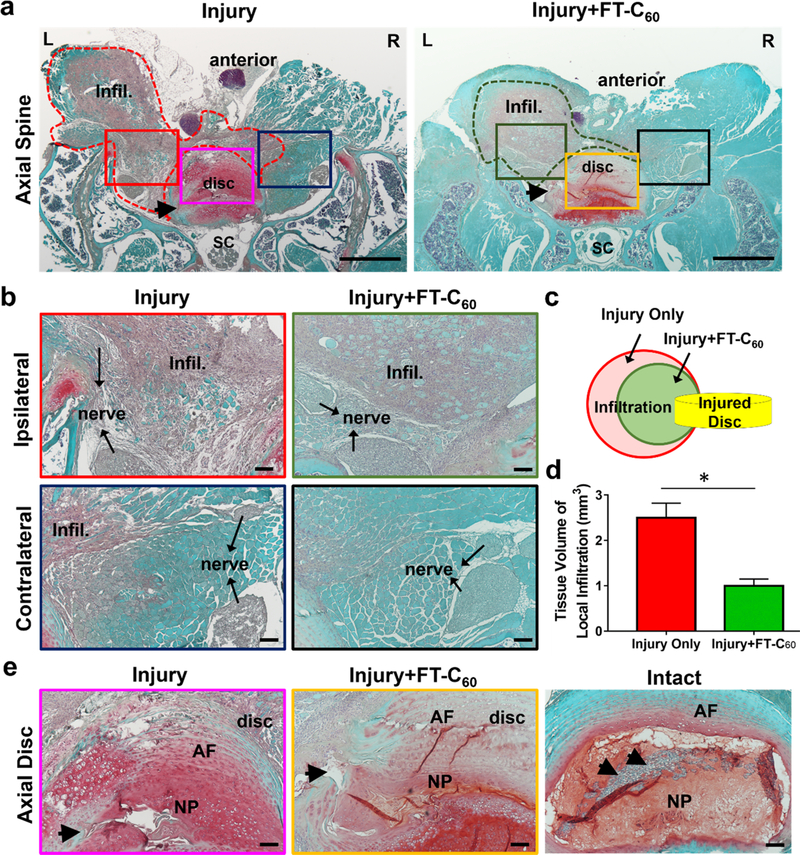
Histological analysis (safranin-O/fast green staining) indicated decreased infiltration tissue volume near the disc puncture site on POD 7. (a) Low (40×)-and (b) high (200×)-magnification axial spine images in the injury group w/ and w/o FT-C60 (10 nmol per 20 g mice, iv) exhibited degenerative disc and abundant inflammatory cells at injury sites close to the exposed nerve root. (c,d) Customized quantitative analysis of infiltration tissue exhibited that systemic FT-C60 reduced the calculated volume of inflammatory tissue. (e) Axial discs in injured groups showed typical signs of degeneration with trajectory of disrupted AF. Infil, inflammatory infiltration; SC, spinal cord; nerve, spinal nerve; AF, annulus fibrosus; NP, nucleus pulposus. (a) Scale bar = 500 μm. (b,e) Scale bar =100 μm.
3. CONCLUSIONS
Existing anti-inflammatory treatments for discogenic pain, such as nonsteroidal anti-inflammatory drug and epidural spinal injection, are problematic in efficacy and safety. Due to repetitive screening and evaluation by various clinical departments, patients have to wait for 7–9 weeks for an injection to be performed by a trained physician under a fluoroscope with an inconsistent outcome. Systemic and target delivery, on the other hand, can be simply delivered via the peripheral vein by a nurse at a clinic or in an emergency room after disc herniation is diagnosed with MRI, leading to reduced medical cost and improved clinical outcome.
Driven by this critically unmet clinical needs, we successfully synthesized and characterized a novel FPR-1 targeting FT-C60 conjugate that possessed superior targeting properties to highly expressed FPR-1 receptors on infiltrated inflammatory cells (such as on macrophages in our study) and promising anti-inflammatory traits of the C60 nanoparticle. We also demonstrated its potent radical scavenging activities via electron paramagnetic resonance spectroscopy. In vitro evaluation of FT-C60 showed its robust anti-inflammatory effects in macrophages with negligible cytotoxicity. In vivo animal studies further depicted effective pain alleviation after a single dose of intravenous injection of FT-C60 in a mouse model of lumbar radiculopathy. Meanwhile, fluorescence molecular imaging confirmed the preferential accumulation and long-lasting half-life in blood circulation given its built-in near-infrared fluorophore. Last but not the least, histological analysis corroborated its anti-inflammatory effects at the tissue level.
As a promising and potential pharmaceutical nanoparticle for discogenic low back pain, the newly synthesized nanoparticle FT-C60 possesses a unique and intrinsic theranostic property during this developmental phase. The specific ligand–receptor binding property of the nanoparticle ensures target delivery by systemic administration. The near-infrared fluorescence tag allows us to visualize and track the drug accumulation and distribution in vivo. Although the fluorophore might be replaced with radioisotope or MRI responsive elements to endow more sensitive detection and translation potential, the increased complexity of the chemistry protocol and altered pharmacokinetic/dynamic profile shall be considered for future translational application. In summary, the successful synthesis and preclinical investigation proved the concept that this novel nanomaterial FT-C60 holds great scientific promise to treat lumbar radiculopathy in a systemic and targeted fashion.
4. EXPERIMENTAL METHODS
4.1. Preparation of the Functionalized C60.
The functionalizetion of the carboxyl groups and hydroxyl groups onto C60 was performed following a previously reported procedure.34 C60 (30 mg) and succinic acid acyl peroxide (49 mg, 5 equiv.) was dissolved in15 mL of o-dichlorobenzene. The resulting solution was deaerated with flowing argon and heated at 84 °C for 120 h. Additional succinic acid acyl peroxide (5 equiv.) was added every 12 h. NaOH (50 mL 0.2 M) was added to the resulting brown sludge to extract the water-soluble product (Figure 2b), resulting in two layers. The top layer, containing the water-soluble C60 derivative, was concentrated and purified via a Sephadex G-25 size-exclusion gel column.
4.2. Chemical Conjugation of FT-C60.
The peptide was conjugated to the functionalized C60 with a microwave-assisted liberty peptide synthesizer. In specific, 0.4 mg of fullerene (0.56 μmol), 0.75 mg of peptide (0.26 μmol), 0.1 mg of O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HATU) (0.26 μmol), and 0.75 μL of DIEA (4.32 μmol) were dissolved in 4 mL of DMSO and allowed to react under microwave for 10 min at 75 °C (CEM liberty 1 microwave system). The resulting solution was filtered through polyacrylamide desalting columns and further purified via HPLC, using a gradient of 10–80% MeCN/0.1% formic acid as the B solvent on a C18 reversed-phase column. Water/0.1% formic acid was used as the A solvent. Collected fractions were characterized with MALDI-TOF mass spectrometry and lyophilized as bluish powder for further characterization and biological tests (see the Supporting Information). The XPS survey spectra were collected by a PHI VersaProbe III scanning XPS microscope, Spectra Research Corporation.
4.3. EPR Detection of Radical Scavenging Capability.
The EPR method was coupled with a spin-trapping agent DEPMPO. Hydroxyl radicals were generated by the classical Fenton reaction, which involves the reaction of FeSO4 (5 mM) and H2O2 (25 mM). DEPMPO (2.5 mM) was used to bind short-lived OH to form a more stable adduct and allow the detection of EPR signals. Water, different concentrations of the functionalized C60, or the conjugate FT-C60 was added to the Fenton reaction solution before the addition of DEPMPO. The EPR assays were carried out at 20 °C using a Bruker ELEXSYS-II EPR spectrometer. Cclear quartz capillary tubes (ID 1.0 mm, OD 1.2 mm) were used as the sample container. The EPR data were collected at ambient temperature, 2 min after initiating the formation of OH by the addition of FeSO4. The following instrument settings were used for collecting EPR spectra: microwave frequency of 9.88 GHz, microwave power of 20.02 mW, field modulation frequency of 100 kHz, and modulation amplitude of 2 G.
To test the superoxide radical anion (O2˙−) scavenging activity, BMPO was used to trap and detect O2˙− by EPR spectroscopy. The superoxide radical anion was generated using the xanthine/xanthine oxidase system. The radical scavenger reaction was initiated by the addition of xanthine oxidase solution (XOD). The reaction contained 20 mM xanthine (1 M NaOH in PBS), 20 mM BMPO, 20 mM DTPA, and 4 U/mL XOD in the presence or absence of the functionalized C60 and the nanoconjugate FT-C60. The EPR spectra were recorded at 2 min after initiating the generation of O2˙− by the addition of XOD. The instrument settings used were the same as hydroxyl radicals.
4.4. In Vitro Cell Viability Assessment.
In brief, Raw 264.7 macrophages were cultured in 96 well plates (tissue culture treated, Thermo Fisher Scientific, Waltham, MA) at 37 °C for 24 h. Cells were cultured with various treatments (fullerol C60-OH (1 μM), carboxyl-C60 (1 μM), peptide-C60 conjugate FT-C60 (25, 50, and 100 nM), and peptide only (1 μM) for 1 and 3 days. At various time points, microscopic images of cells were taken at a magnification of 200×. At the end of experiments, cells were lysed with 0.1% Triton X-100 in PBS. The DNA assay was performed with a Hoechst dye and measured on a Hoefer DyNA Quant 200 spectrometer using calf thymus DNA as a standard.10 Four repeated measurements were performed for each sample, and three biological replicate samples were tested for each treatment condition. The Hoechst dye enhanced fluorescence signal of each group was normalized to the value of the untreated control group at all time points.
4.5. In Vitro Binding of FT-C60 to Macrophages.
Raw 264.7 macrophages were seeded on a Lab-Tek 16 well chamber (Thermo Fisher Scientific, Waltham, MA) at a density of 1 × 105 cells per well in 0.5 mL of complete growth media at 37 °C overnight. Cells were treated with or without LPS (100 ng/mL) for 5 h. Then cell media was replaced with fresh serum-free DMEM containing either peptide 1 (1 μM) or FT-C60 (100 nM) in both control and LPS treated groups and incubated at 37 °C for another 30 min. Cells were then fixed with 4% paraformaldehyde (PFA) for 10 min at room temperature. Cells were washed with PBS and mounted with Vectashield antifade mounting media (w/ DAPI) (Vector Laboratories, Burlingame, CA). Fluorescence images were taken with a confocal fluorescence microscope (LSM 510-UV, Carl Zeiss, Germany) and processed by LSM Image Browser software (Carl Zeiss, Germany).30
4.6. Cell Treatment, RNA Isolation, Reverse Transcription, and Real-Time Reverse Transcription Polymerase Chain Reaction.
Raw 264.7 cells were seeded onto 48 well plates at a density of 1 × 105 cells/mL and cultured for 1–2 days until 90% confluency. Cells were pretreated with peptide 1 (1 μM), functionalized C60 (1 μM), or FT-C60 (25, 50, 100 nM) in serum-free DMEM for 20 h and then stimulated with LPS (100 ng/mL) for 5 h. Total RNA was isolated with the Trizol reagent (Invitrogen) and quantified with a NanoDropTM 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The cDNA was subsequently synthesized with the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) using 1 μg of total RNA following the manufacturer’s instruction. Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed with an RT̂2 SYBR Green Fluo FAST Mastermix (Qiagen Sciences, Germantown, MA) and a QuantStudio3 Real-time PCR system (Applied Biosystems by Thermo Fisher Scientific). Each qPCR reaction solutions were prepared by 10 ng of cDNA, 5 μM desired primer, and SYBR Premix Ex Taq (1×) in a total volume of 12.5 μL with 35 cycles. The mRNA expressions of IL-6, IL-1, COX-2, iNOS, and TNF-α were evaluated. The 18S rRNA and GAPDH were used as internal controls. Sequences of primers, individual annealing temperature, and amplicon lengths were the same as previously reported and are listed in Table S1.
4.7. Mouse Disc Herniation Induced Radiculopathy.
B6 mice were purchased from Charles River (Indianapolis, IN, USA, 8–10 weeks old, male, 20–25 g). Animal use was approved by the Institutional Animal Care and Use Committee at the University of Virginia. General anesthesia was induced with intraperitoneal injection of ketamine/xylazine (60–80/5–10 mg/kg). Briefly, using aseptic techniques and a surgical microscope, the spine was exposed through an anterior midline transperitoneal approach. After separating the hind peritoneum and psoas major muscle, the L4 and L5 vertebral bodies were identified. The left L5 nerve root was exposed by removing the overlying psoas muscle fibers, and the L4–5 disc was punctured laterally to enable nucleus protrusion toward the L5 nerve root. Animals were randomly assigned to three groups: injured (n = 12) (treated with saline), local C60 (n = 12), and systemic FT-C60 (n = 12). For the injured group, physiological saline (10 μL) was added dropwise onto the herniated disc puncture. For the local C60 group, freshly prepared fullerol C60 solution (10 μL, 1μM in distilled and deionized water) was applied dropwise using a syringe onto the herniated disc. For the systemic nanoconjugate group, 10 nmol per 20 g of mice of FT-C60 in saline was intravenously injected via the tail vein 1–2 h after mice woke up from surgeries. A sham-operated group (n = 5) with nerve exposure but without a disc puncture was performed prior to the above surgeries to establish a baseline for mechanical hyperalgesia. The sham-operated group exhibited unchanged baselines as previously published by us15,20,31.
4.8. Electronic von Frey Test.
To assess the mechanical sensitivity of animals subjected to disc herniation with or without treatment, the electronic von Frey test was performed on both hind paws for 3 consecutive days prior to surgery and every other day up to POD 12 in a blinded fashion, following reported protocol.31,32 In brief, mice were acclimated on an elevated mesh grid, and the hind paw withdrawal threshold was determined using the electronic von Frey apparatus.35 Five trials were conducted on each paw, with at least 5 min of rest time between trials on opposite paws and 10 min between trials on the same paw. For each paw, the mechanical withdrawal thresholds from five trials were averaged after excluding the maximum and minimum reading. Both ipsilateral and contralateral mechanical thresholds of all groups were measured, and the mean threshold value of each group was averaged at each time point.
4.9. Ex Vivo Near-Infrared Fluorescence Imaging of Mouse Spine and Organs.
At 1 and 2 weeks of post-surgery, mice were euthanized with 100% CO2. Lumbar spines were harvested under a surgical microscope to locate the injured disc level. Ex vivo imaging of lumbar spines and organs (heart, liver, spleen, lung, kidneys, muscle, brain, bone, brain, and fat) from all groups was performed with the epifluorescence function using Xenogen IVIS Spectrum and Live Image software.14,33,36–40
4.10. Histological Staining.
Lumbar spines were fixed in 10% formalin, decalcified in 0.25 M ethylenediaminetetraacetic acid (EDTA) for 2 weeks, embedded in paraffin, and sectioned either trans-axially or mid-sagittally (5 μm thickness). Three sections were collected on each glass slide. For axial section samples, safranin-O (0.1%) staining was performed on every five slides to detect the disc degenerative change to visualize both injured disc and anterior inflammatory cell infiltration on the same plane. Visual examination under a microscope was carefully conducted to locate the entire range of spine sections encompassing the injured disc level and anterior inflammatory infiltration areas, as this was a critical step to evaluate severity of inflammation in each group. For sagittal sections, alcian blue/picrosirious red staining was performed using 1% alcian blue solution (pH 2.5) and picrosirius red (0.1% sirius red in saturated aqueous picric acid) for alcian blue/picrosirius red staining following the published protocol.14,15,31
4.11. Quantification of Cell Infiltration.
All microscopic images were taken by a Nikon ECLIPSE E600 with Nikon Elements. Representative images of fields of interest were taken at magnifications of 40×, 100×, and 200× to visualize both gross anatomic structure and cell composition details. At a magnification of 100×, all areas of anterior inflammatory infiltration of every stained slide (one image was taken every 15 sections) were recorded first and then subjected to quantitative analyses. First of all, the color hue setting was set to 64 to provide the best contrast. Free-draw regions of interest (ROI) that adequately included the entire quantifiable inflammatory cell infiltration and excluded the large, blank, bony, and disc area was applied and analyzed. A previously optimized object count threshold for area quantification (brown and blue in this case) was loaded and kept consistent throughout quantification. The analysis output from built-in software for each ROI contained a total area (total area of selected ROI) and a color intensity ratio of brown to blue. The color intensity ratio of brown to blue was calculated for each group, representing the area percentage of inflammatory tissue to total ROI. For each ROI, the area of inflammatory tissue was obtained by total area times the percentage. An area Atrans (infiltration area of trans-axial images on every 15 slides) value was obtained by summing up infiltration areas of all ROIs. For each mouse lumbar spine sample, ~300 trans-axial sections were collected to cover the entire affected disc with inflammatory infiltrations in the surroundings. Each Atrans represented different values due to the irregular shape of inflammatory cell infiltration. A small volume of inflammation tissue Vsmall was then calculated by (given the tissue between #30 to #45 as an example) 0.5 × (infiltration area of section #30 + infiltration area of section #45) × 15 sections × 5 μm/section (1)
The total volume of inflammatory tissue Vinfil for each spinal sample was then obtained by adding up all Vsmall. Three to four mouse spinal samples were analyzed per group.
4.12. Statistical Analysis.
All in vitro experiments were performed in triplicate. Quantitative data were presented as mean ± SEM. One-way ANOVA and multiple comparisons were used to analyze PCR and pain data using GraphPad Prism. Data comparing two groups were analyzed with Student’s t test. A p value less than 0.05 was considered as statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to financial support from US NIH NIAMS R01AR064792, R21AR057512, Commonwealth Health Research Board (CHRB), Orthopaedic Trauma Association, and North American Spine Society (NASS), and start-up fund from the Department of Orthopaedic Surgery at the University of Virginia.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.9b11783.
Detailed materials and reagents, synthesis and characterization of targeting peptides (Scheme S1, Figures S1, S2), intermediate synthesis of functionalized C60 (Scheme S2). Characterization of the functionalized C60 and the peptide-C60 conjugate FT-C60 including XPS multiplex, MALDO-TOF-MS, 1H NMR, gel electrophoresis, UV–vis spectroscopy, and partition coefficient (Figures S3–S7). Cell culture and immunofluorescence staining of FPR-1 in macrophage cells (Figure S8). Macrophage cell morphology under dose-dependent treatment of FT-C60 (Figure S9). Time-dependent LPS stimulation of proinflammatory genes (Figure S10). Real-time RT-PCR of pro-inflammatory genes normalized by 18S (Figure S11). Unchanged proinflammatory gene profile of macrophages with peptide 1 treatment by real-time RT-PCR (Figure S12). Additional ex vivo NIRF imaging of lumbar spine (Figure S13) and representative organ distribution images (Figure S14). Additional histological images (Figure S15) and customized method for histological quantification (Figure S16). Primer sequences of real-time RT-PCR experiments (Table S1) (PDF) A mouse treadmill video of pain behavior on POD 6 showing in vivo efficacy of systemic delivery of FT-C60 (MP4)
REFERENCES
- (1).Freburger JK; Holmes GM; Agans RP; Jackman AM; Darter JD; Wallace AS; Castel LD; Kalsbeek WD; Carey TS The Rising Prevalence of Chronic Low Back Pain. Arch. Intern. Med 2009,169, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hurwitz EL; Randhawa K; Yu H; Cote P; Haldeman S. The Global Spine Care Initiative: a Summary of the Global Burden of Low Back and Neck Pain Studies. Euro. Spine J 2018, 27, 796–801. [DOI] [PubMed] [Google Scholar]
- (3).Balague F; Mannion AF; Pellise F; Cedraschi C. Non-specific Low Back Pain. Lancet 2012, 379, 482–91. [DOI] [PubMed] [Google Scholar]
- (4).Schwarzer AC; Aprill CN; Derby R; Fortin J; Kine G; Bogduk N. The Prevalence and Clinical Features of Internal Disc Disruption in Patients with Chronic Low Back Pain. Spine 1995, 20, 1878–1883. [DOI] [PubMed] [Google Scholar]
- (5).Goupille P; Jayson MIV; Valat J-P; Freemont AJ The Role of Inflammation in Disk Herniation-associated Radiculopathy. Semin. Arthritis Rheum 1998, 28, 60–71. [DOI] [PubMed] [Google Scholar]
- (6).Molinos M; Almeida CR; Caldeira J; Cunha C; Goncalves RM; Barbosa MA Inflammation in Intervertebral Disc Degeneration and Regeneration. J. R. Soc., Interface 2015, 12, 20141191–20141191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Koes BW; van Tulder MW; Peul WC Diagnosis and Treatment of Sciatica. BMJ 2007, 334, 1313–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hughes SPF; Freemont AJ; Hukins DWL; McGregor AH; Roberts S. The Pathogenesis of Degeneration of the Intervertebral Disc and Emerging Therapies in the Management of Back Pain. J. Bone Joint Surg. Br 2012, 94-B, 1298–1304. [DOI] [PubMed] [Google Scholar]
- (9).Adams MA; Roughley PJ What is Intervertebral Disc Degeneration, and What Causes It? Spine 2006, 31, 2151–61. [DOI] [PubMed] [Google Scholar]
- (10).Xiao L; Ding M; Saadoon O; Vess E; Fernandez A; Zhao P; Jin L; Li X. A Novel Culture Platform for Fast Proliferation of Human Annulus Fibrosus Cells. Cell Tissue Res. 2017, 367, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Rothoerl RD; Woertgen C; Brawanski A. Pain Resolution After Lumbar Disc Surgery is Influenced by Macrophage Tissue Infiltration. A prospective Consecutive Study on 177 patients. J. Clin. Neurosci 2002, 9, 633–636. [DOI] [PubMed] [Google Scholar]
- (12).Hamamoto H; Miyamoto H; Doita M; Takada T; Nishida K; Kurosaka M. Capability of Nondegenerated and Degenerated Discs in producing inflammatory agents with or without macrophage interaction. Spine 2012, 37, 161–167. [DOI] [PubMed] [Google Scholar]
- (13).Takada T; Nishida K; Maeno K; Kakutani K; Yurube T; Doita M; Kurosaka M. Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis Rheum. 2012, 64, 2601–2610. [DOI] [PubMed] [Google Scholar]
- (14).Xiao L; Ding M; Zhang Y; Chordia M; Pan D; Shimer A; Shen F; Glover D; Jin L; Li X. A Novel Modality for Functional Imaging in Acute Intervertebral Disk Herniation via Tracking Leukocyte Infiltration. Mol. Imaging Biol 2017, 19, 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Jin L; Ding M; Oklopcic A; Aghdasi B; Xiao L; Li Z; Jevtovic-Todorovic V; Li X. Nanoparticle fullerol alleviates radiculopathy via NLRP3 inflammasome and neuropeptides. Nanomedicine: NBM 2017, 13, 2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chen BX; Wilson SR; Das M; Coughlin DJ; Erlanger BF Antigenicity of fullerenes: antibodies specific for fullerenes and their characteristics. Proc. Natl. Acad. Sci U. S. A 1998, 95, 10809–10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Li T; Xiao L; Yang J; Ding M; Zhou Z; LaConte L; Jin L; Dorn HC; Li X. Trimetallic Nitride Endohedral Fullerenes Carboxyl-Gd3N@C80: A New Theranostic Agent for Combating Oxidative Stress and Resolving Inflammation. ACS Appl. Mater. Interfaces 2017, 9, 17681–17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Xiao L; Li T; Ding M; Yang J; Rodríguez-Corrales J; LaConte SM; Nacey N; Weiss DB; Jin L; Dorn HC; Li X. Detecting Chronic Post-Traumatic Osteomyelitis of Mouse Tibia via an IL-13Ra2 Targeted Metallofullerene Magnetic Resonance Imaging Probe. Bioconjugate Chem. 2017, 28, 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Roy P; Bag S; Chakraborty D; Dasgupta S. Exploring the Inhibitory and Antioxidant Effects of Fullerene and Fullerenol on Ribonuclease A. ACS omega 2018, 3, 12270–12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Xiao L; Hong K; Roberson C; Ding M; Fernandez A; Shen F; Jin L; Sonkusare S; Li X. Hydroxylated Fullerene: A Stellar Nanomedicine to Treat Lumbar Radiculopathy via Antagonizing TNF-α-Induced Ion Channel Activation, Calcium Signaling, and Neuro-peptide Production. ACS Biomater. Sci. Eng 2018, 4, 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Liu Q; Jin L; Mahon BH; Chordia MD; Shen FH; Li X. Novel Treatment of Neuroinflammation Against Low Back Pain By Soluble Fullerol Nanoparticles. Spine 2013, 38, 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Mandal P; Novotny M; Hamilton TA Lipopolysaccharide Induces Formyl Peptide Receptor 1 Gene Expression in Macrophages and Neutrophils via Transcriptional and Posttranscriptional Mechanisms. J. Immunol 2005, 175, 6085–6091. [DOI] [PubMed] [Google Scholar]
- (23).Thomas M; Klibanov AM Conjugation to Gold Nanoparticles Enhances Polyethylenimine’s Transfer of Plasmid DNA Into Mammalian Cells. Proc. Natl. Acad. Sci. U. S.A 2003, 100, 9138–9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Chompoosor A; Saha K; Ghosh PS; Macarthy DJ; Miranda OR; Zhu Z-J; Arcaro KF; Rotello VM The Role of Surface Functionality on Acute Cytotoxicity, ROS Generation and DNA Damage by Cationic Gold Nanoparticles. Small 2010, 6, 2246–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Wuertz K; Haglund L. Inflammatory Mediators in Intervertebral Disk Degeneration and Discogenic Pain. Global Spine J. 2013, 3, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Duque GA; Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol 2014, 5,491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Meng F; Lowell CA Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med 1997, 185, 1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Gillette DD; Tridandapani S; Butchar JP Monocyte/ macrophage Inflammatory Response Pathways to Combat Francisella Infection: Possible Therapeutic Targets? Front. Cell. Infect. Microbiol 2014, 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Fang H; Pengal RA; Cao X; Ganesan LP; Wewers MD; Marsh CB; Tridandapani S. Lipopolysaccharide-Induced Macrophage Inflammatory Response Is Regulated by SHIP. J. Immunol 2004, 173, 360–366. [DOI] [PubMed] [Google Scholar]
- (30).Andreakos E; Sacre SM; Smith C; Lundberg A; Kiriakidis S; Stonehouse T; Monaco C; Feldmann M; Foxwell BM Distinct Pathways of LPS-induced NF-kB Activation and Cytokine Production in Human Myeloid and Nonmyeloid Cells Defined by Selective Utilization of MyD88 and Mal/TIRAP. Blood 2004, 103, 2229–2237. [DOI] [PubMed] [Google Scholar]
- (31).Xiao L; Ding M; Fernandez A; Zhao P; Jin L; Li X. Curcumin Alleviates Lumbar Radiculopathy by Reducing Neuro-inflammation, oxidative stress and nociceptive factors. Euro. Cell. Mater 2017, 33, 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Martinov T; Mack M; Sykes A; Chatterjea D. Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von Frey apparatus. J. Vis. Exp. 2013, e51212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Xiao L; Zhang Y; Berr SS; Chordia MD; Pramoonjago P; Pu L; Pan D. A Novel Near-Infrared Fluorescence Imaging Probe for in Vivo Neutrophil Tracking. Mol. Imaging 2012, 11, 372–382. [PubMed] [Google Scholar]
- (34).Shu C; Corwin FD; Zhang J; Chen Z; Reid JE; Sun M; Xu W; Sim JH; Wang C; Fatouros PP; Esker AR; Gibson HW; Dorn HC Facile preparation of a new gadofullerene-based magnetic resonance imaging contrast agent with high 1H relaxivity. Bioconjugate Chem. 2009, 20, 1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Bonin RP; Bories C; De Koninck Y. A Simplified Up-Down Method (SUDO) for Measuring Mechanical Nociception in Rodents Using von Frey Filaments. Mol. Pain 2014, 10, 1744–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Guan Y; Zhang Y; Xiao L; Li J; Wang J-P; Chordia MD; Liu Z-Q; Chung LWK; Yue W; Pan D. Improving Therapeutic Potential of Farnesylthiosalicylic Acid: Tumor Specific Delivery via Conjugation with Heptamethine Cyanine Dye. Mol. Pharmaceutics 2017, 14, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Zhang Y; Xiao L; Popovic K; Xie X; Chordia MD; Chung LWK; Williams MB; Yue W; Pan D. Novel Cancer-Targeting SPECT/NIRF Dual-modality Imaging Probe 99mTc-PC-1007: Synthesis and Biological Evaluation. Bioorg. Med. Chem. Lett. 2013, 23, 6350–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Xiao L; Zhang Y; Yue W; Xie X; Wang J-P; Chordia MD; Chung LWK; Pan D. Heptamethine Cyanine Based 64Cu-PET Probe PC-1001 for Cancer Imaging: Synthesis and In vivo Evaluation. Nucl. Med. Biologia 2013, 40, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zhang Y; Xiao L; Chordia MD; Locke LW; Williams MB; Berr SS; Pan D. Neutrophil Targeting Heterobivalent SPECT Imaging Probe: cFLFLF-PEG-TKPPR-99mTc. Bioconjugate Chem. 2010, 21, 1788–1793. [DOI] [PubMed] [Google Scholar]
- (40).Xiao L; Zhang Y; Liu Z; Yang M; Pu L; Pan D. Synthesis of the Cyanine 7 Labeled Neutrophil-specific Agents for Noninvasive Near Infrared Fluorescence Imaging. Bioorg. Med. Chem. Lett. 2010, 20, 3515–3517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.