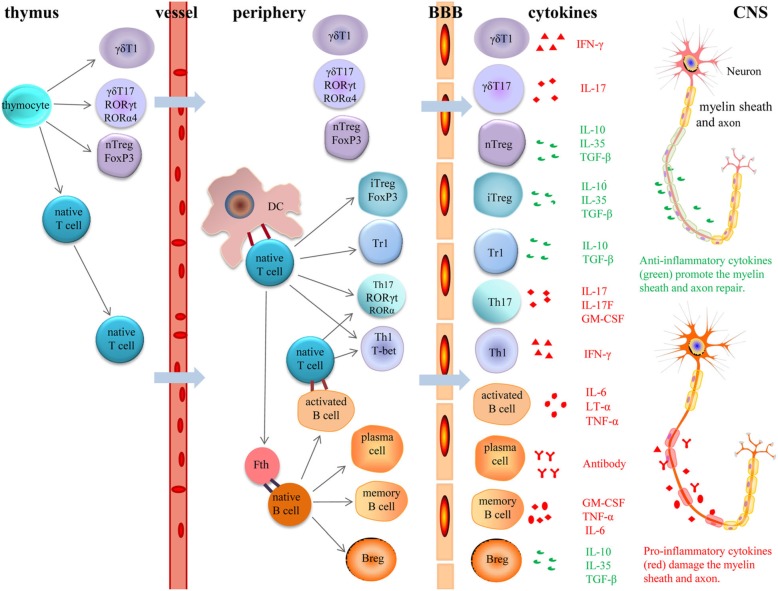
Fig. 2.
Role of different cells in the pathogenesis of MS and EAE. In the thymus, thymocyte precursor cells develop into γδT1, γδT17, nTregs and naïve CD4+ T cells. Upon neuroinflammation, γδT1 and γδT17 cells can cross the endothelial BBB and traffic into the central nervous system CNS, whereas naïve T cells migrate into the peripheral immune tissue. Naïve T cells connected with APCs (DCs and B cells), thereby differentiating into various effector T cells (iTregs, Tr1, Th17 and Th1). Th1, Th17, γδT1 and γδT17 cells secrete pro-inflammatory cytokines that trigger neuroinflammation and impair the myelin sheath and axons. Meanwhile, Tregs (Tr1, iTregs and nTregs) secrete anti-inflammatory cytokines and restrain immune responses mediated by T cells, B cells and DCs, thereby promoting tissue repair. Further, with the help of Tfh cells, naïve B cells differentiate into plasma cells, memory B cells and Bregs. While plasma cells damage the myelin sheath and axons on neurons via secreting antibodies, Bregs play a protective role via producing IL10, IL35 and TGF-β. Memory B cells and several activated B cells can produce a series of pathogenic cytokines
Abbreviations: MS: multiple sclerosis; EAE: experimental autoimmune encephalomyelitis; γδ: gamma delta; BBB: blood-brain barrier; CNS: central nervous system; APC: professional antigen presenting cells; DC: dendritic cell; iTreg: induced regulatory T cell; Tr1: type 1 Treg; Th: T helper; nTreg: natural regulatory T cell; Tfh: follicular helper T; Breg: regulatory B cell; IL: interleukin; IFN-γ: interferon-γ; TGF-β: transforming growth factor-β; TNF-α: tumor necrosis factor α; GM-CSF: granulocyte monocyte-colony stimulating factor