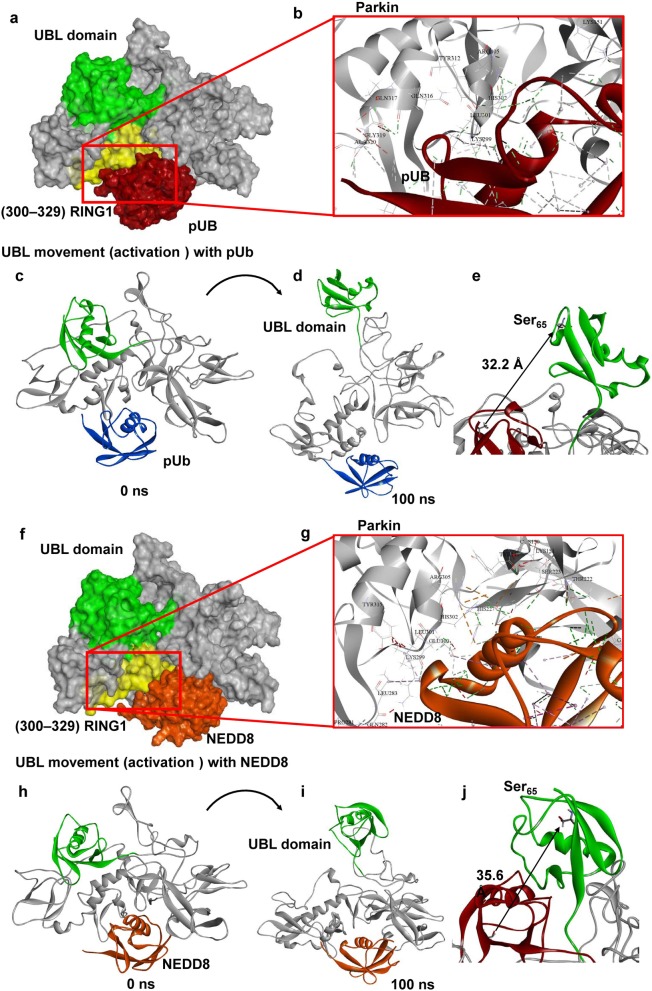
Fig. 5.
Molecular modeling indicates NEDD8 binds to parkin in the same position as P-Ub and opens up the UBL domain in a manner similar to P-Ub. a Molecular interactions for P-Ub with parkin at the P-Ub binding helix agree with the previous experimental model of P-Ub interaction with parkin [1] (b). All-atom metadynamics simulation of the P-Ub-parkin complex predicts the movement of the UBL domain exposing the Ser65 from the auto-inhibited form of parkin (c–e). Protein-protein docking predicts NEDD8 interacts with the auto-inhibited form of parkin similar to that of P-Ub (f). Molecular interaction analysis indicates that NEDD8 binds to parkin at the same location as P-Ub, i.e., at the P-Ub binding helix (g). All-atom metadynamics simulation of the NEDD8-parkin complex, like that of P-Ub, also opened the UBL domain, exposing Ser65 (h–j)