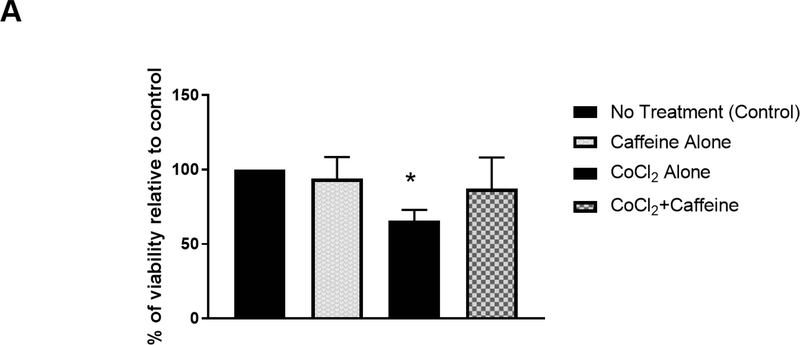
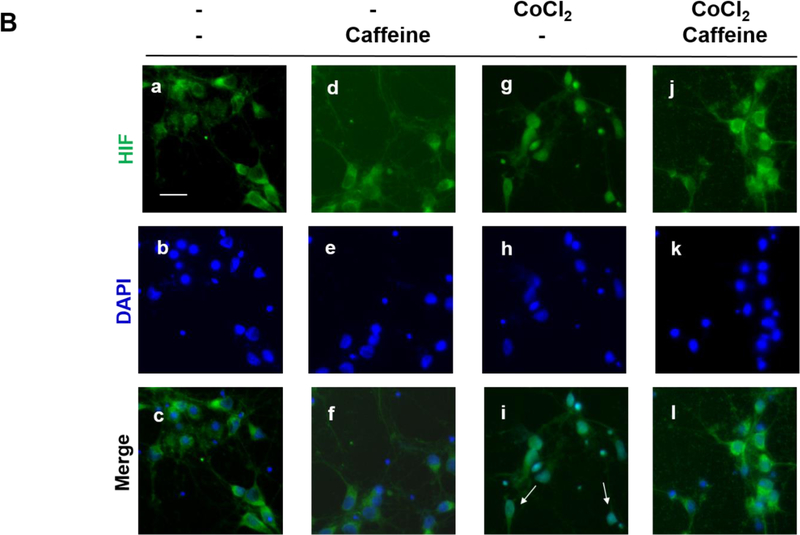
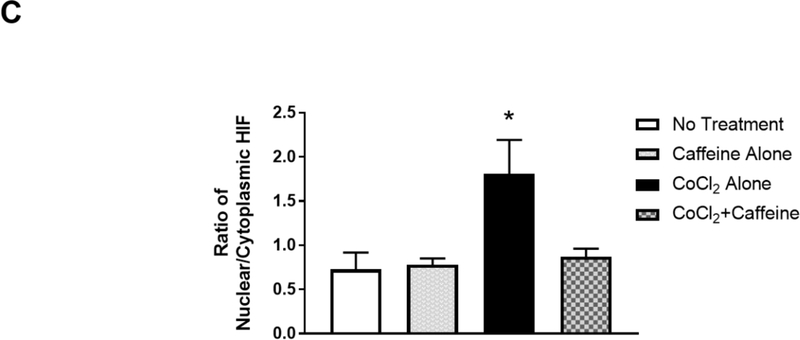
Figure 3.
Caffeine promoted cell survival after hypoxia and decreased CoCl2-induced nuclear accumulation of HIF-1α in vitro. (A) Primary cortical neuronal cultures were treated with CoCl2 or vehicle (control) for 24 h. Each group was divided into two subgroups that were either treated with 100 μM caffeine or vehicle for an additional 24 h. The viability of each treatment group was subsequently measured by MTT assays. Data are presented as percentage of viability in each group relative to that of the control group (control, 100%; caffeine alone, 94±7.1%; CoCl2 treatment, 65.6±3.6%; CoCl2+caffeine, 87.2±10.5% n=4. p<0.001).
(B) Treated neurons were double-immunostained to label HIF-1α (green, top row) and neuron-specific beta-III tubulin (red, not shown) and counter-stained with DAPI for nuclei (blue, middle row). The merged images of HIF-1α and DAPI are shown in the bottom row. Beta-III tubulin immunostaining (not shown) was used as a neuronal marker. In the absence of CoCl2, HIF-1α was primarily localized in the cytoplasm regardless of whether neurons received caffeine or not (Lane 1, a, b, c and Lane 2, d, e, f). In contrast, CoCl2 triggered nuclear co-localization of HIF-1α with DAPI (Lane 3, g.h.i), suggesting nuclear accumulation of HIF-1α. White arrows indicate co-localization of HIF-1α and DAPI. This nuclear accumulation of HIF-1α was alleviated by subsequent treatment with caffeine (Lane 4, j, k,l). Scale bar, 20 μm. (C) Quantification of the ratio of nuclear vs cytoplasmic HIF-1α in neurons of each treatment group (control, 0.73±0.07; caffeine alone, 0.78±0.03; CoCl2 treatment, 1.81±0.17, CoCl2 and caffeine, 0.82±0.04, p<0.001, n=5).