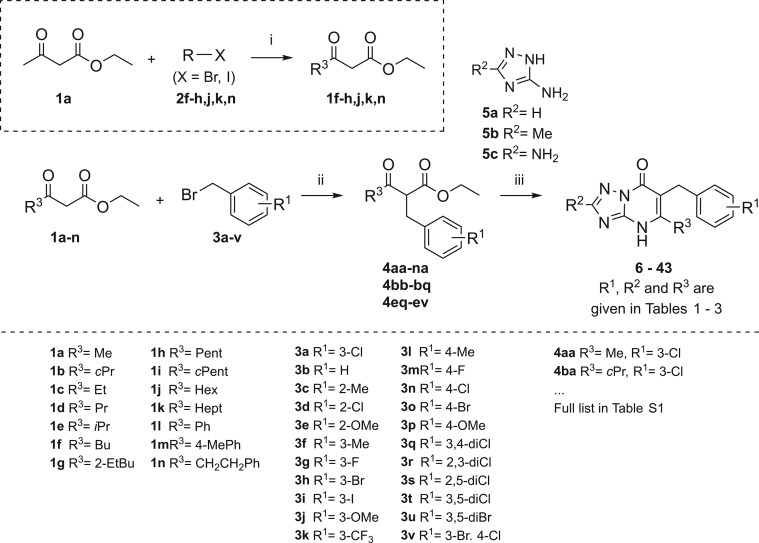
Scheme 1. Synthesis Scheme of the Triazolopyrimidinone Derivatives 6–43.
Reagents and conditions: (i) NaH, n-BuLi, THF, overnight, 0 °C to rt (1a–e,i,l,m were commercially available); (ii) DIPEA, LiCl, THF, reflux, overnight; (iii) (8–43, R2 = NH2) BMIM-PF6, 200 °C, 1 h or (6, R2 = H) H3PO4, EtOH, 170 °C, 10 h or (7, R2 = Me) p-toluenesulfonic acid monohydrate, 180 °C, 30 min.