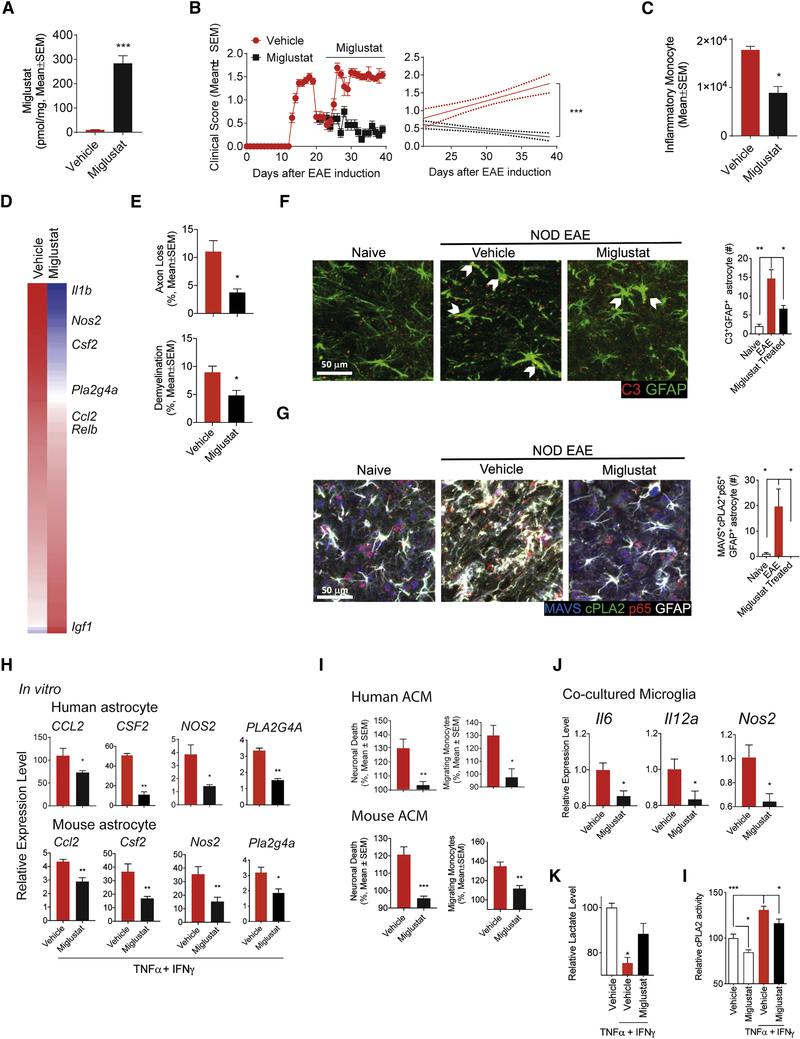
Figure 6. Miglustat ameliorates chronic progressive NOD EAE.
(A) Miglustat levels in the CNS after oral administration of 600 mg/kg Miglustat. (unpaired T test) (B) EAE development in NOD mice treated with Miglustat (600 mg/kg administered orally before initiation of progressive phase, n≥6, N=3, Regression slope T test). (C-G) CNS samples were harvested 41 days after EAE induction from mice treated with Miglustat or vehicle as shown in Figure 6b. The number of CNS-infiltrating inflammatory monocytes (C, unpaired T test). Whole genome expression in astrocytes isolated from Miglustat treated NOD EAE mice (D; n = 6). Axonal loss and demyelination in spinal cord (E, unpaired T test). Immunoflourescence analysis of C3+GFAP+ astrocytes. Bar plots depict the number of C3+GFAP+ astrocyte within the observation field (F, unpaired T test, compared to EAE condition). Immunofluorescence analysis of NF-κB activation among MAVS+cPLA2+GFAP+ astrocytes (G, unpaired T test, compared to EAE condition). Bar plots depict the number of MAVS+cPLA2+acetyl-p65+GFAP+ astrocyte within the observation field. (H) mRNA expression determined by qPCR in activated human and mouse astrocytes in the presence of Miglustat. Data are shown relative to resting astrocytes. (unpaired T test) (I) Human and mouse astrocyte conditioned medium were analyzed using in vitro monocyte migration and neurotoxicity assays. Migrating monocytes and neuronal death in the resting vehicle-treated group were set as 100%. (unpaired T test) (J) mRNA expression in polarized microglia was determined by qPCR. Microglia was co-cultured with activated astrocytes. (unpaired T test) (K) Lactate release from activated astrocytes in the presence of Miglustat. (unpaired T test, compared to activated vehicle condition) (L) Effect of Miglustat treatment on cPLA2 enzymatic activity in astrocytes, evaluated by cPLA2 activity assay. (unpaired T test, compared to all)