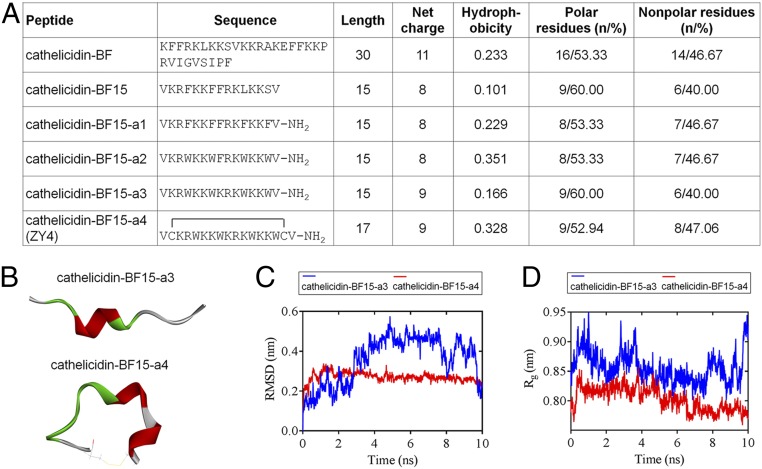
Fig. 1.
Primary sequence and structure prediction of ZY4 and its analogs. (A) Sequence and physicochemical properties of the designed peptides. (B) The 3-dimensional structures represent the last frames (t = 10 ns) of cathelicidin-BF15-a3 and cathelicidin-BF15-a4. The different colors represent various secondary structure types: green, turn; red, helix; white, coil. The yellow lines in cathelicidin-BF15-a4 indicate the disulfide bond. (C) The cathelicidin-BF15-a4 with lower and steady rmsd levels indicates that the structure of ZY4 is stabler than the structure of cathelicidin-BF15-a3. (D) The lower and steady Rg values of cathelicidin-BF15-a4 indicate that after introducing the disulfide bond, cathelicidin-BF15-a4 was stabler than cathelicidin-BF15-a3.