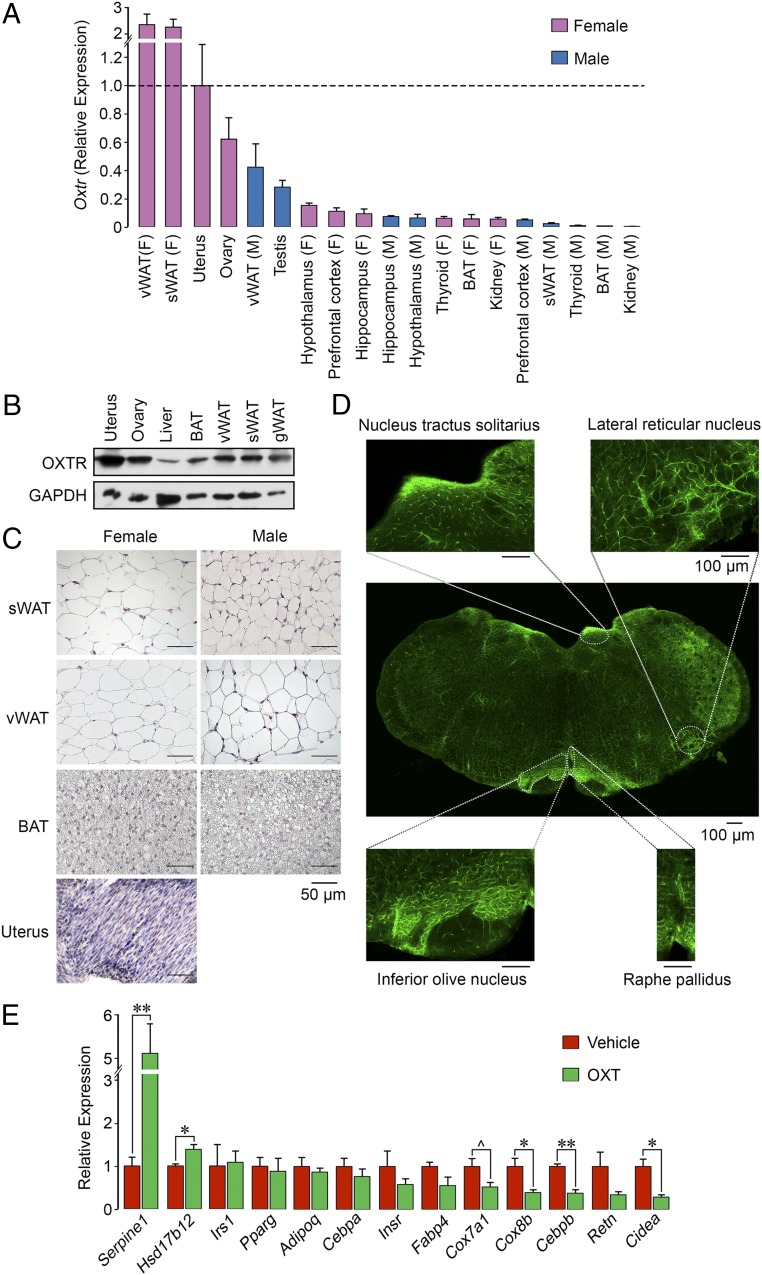
Fig. 4.
Oxytocin acts on adipocyte oxytocin receptors to suppress beiging. (A) qPCR showing the expression of OXTR in mouse tissues of interest, including the uterus, ovary, testes, 3 brain regions, and visceral (v) and subcutaneous (s) WAT and BAT. (B) Western blot analysis using an anti-OXTR antibody (Abcam; ab181077) showed OXTR protein expression in female uterus, ovary, liver, BAT, and visceral, subcutaneous, and perigonadal WAT. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as a loading control. (C) Immunohistochemistry using the same anti-OXTR antibody confirming high expression levels in uterus, with a strong signal in subcutaneous and visceral WAT and limited expression in BAT. (Scale bar: 50 µm.) (D) Immunofluorescence images of OXTR expression in various brain regions, including the nucleus tractus solitarius, lateral reticular nucleus, inferior olive nucleus, and raphe pallidus. The whole brain section was composed from 12 images. (Scale bar: 100 µm.) (E) qPCR on RNA isolated from adipocytes derived from precursor 3T3.L1 cells following a 14-d incubation in differentiation medium and a 2-d incubation with OXT. There was a significant (*P < 0.05, **P < 0.01) reduction in or a trend toward reduced expression (^0.05 < P < 0.1) of certain genes involved in beiging, namely Cox7a, Cox8b, Cebpb, Retn, and Cidea. Certain genes involved in steroidogenesis and thrombosis, namely Hsd17b12 and Serpine1, respectively, were up-regulated. The qPCR data were normalized to housekeeping genes Actb (A) Gapdh (A and D), Rps11 (A and D), and Tuba1a (A and D). Three biological replicates with 3 technical replicates were used. Data are expressed as mean ± SEM; comparisons with vehicle treatment, 2-tailed Student’s t test.