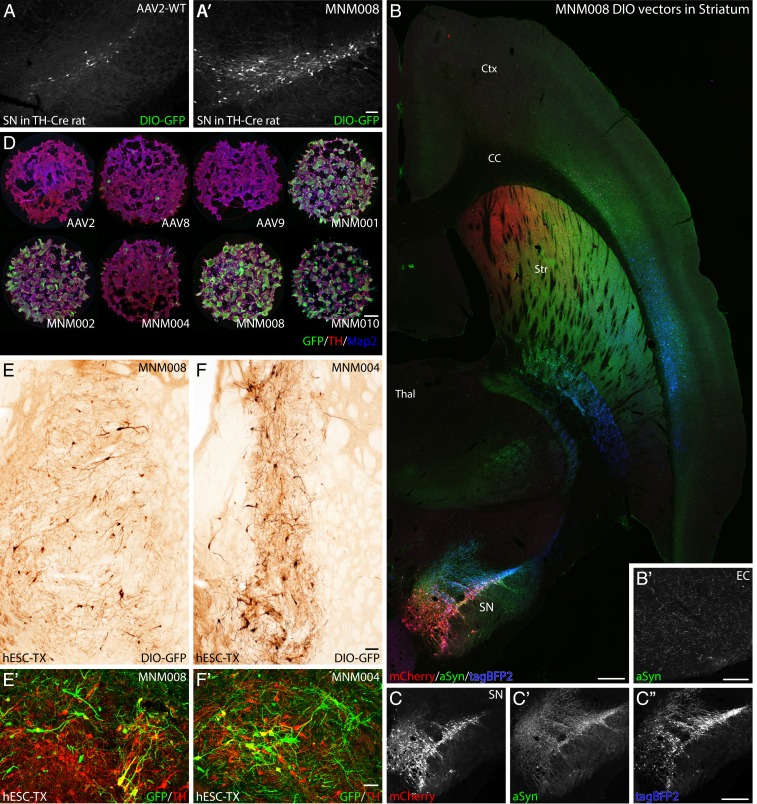
Fig. 4.
Validation of AAV capsids infecting rat and human dopaminergic neurons in vivo and in vitro. (A and A′) In vivo comparison between the CAV-2–derived MNM008 and AAV2-WT assessing infectivity of dopamine neurons from their terminals in the striatum. Both vectors express Cre-inducible GFP (double-floxed inverted orientation, DIO-GFP) and were injected into the striatum of TH-Cre knockin rats. (B and C″) Mapping of nigral afferent topography to the striatum using 3 Cre-inducible genes (mCherry, red; alpha-synuclein, green; tagBFP2, blue) packaged in separate MNM008 vectors and injected into the striatum along the rostrocaudal axis (B), as illustrated in a horizontal section including both striatum (Str) and substantia nigra pars compacta (SN) imaged using a laser-scanning confocal microscope. (C–C″) The nigro-striatal topology is visible in the transgene expression pattern in the SN. (B′) transduced DA-neurons send collaterals as far back as the entorhinal cortex (EC). (D) Assessment of retained neuronal tropism in human embryonic stem cell-derived DA neuroblasts in vitro. (E and F) Assessment of retrograde infectivity in humanized rats. These animals first received an hESC-derived DA-rich neuronal transplant (expressing Cre) into the striatum. Six months later, the MNM008 or MNM004 vectors (expressing DIO-GFP) were injected into the frontal cortex. hESC-derived neurons in the graft were efficiently labeled by both vectors (E and F) and the vast majority expressed the DA neuron marker tyrosine hydroxylase (TH) determined using confocal microscopy (E′ and F′). (Scale bars: in A′ represents 100 µm in A and A′; in B represents 500 µm in B, C, and C″; in B′ represents 100 µm; in D represents 100 µm; in E represents 50 µm in E and F; and in E′ represents 50 µm in E′ and F′.)