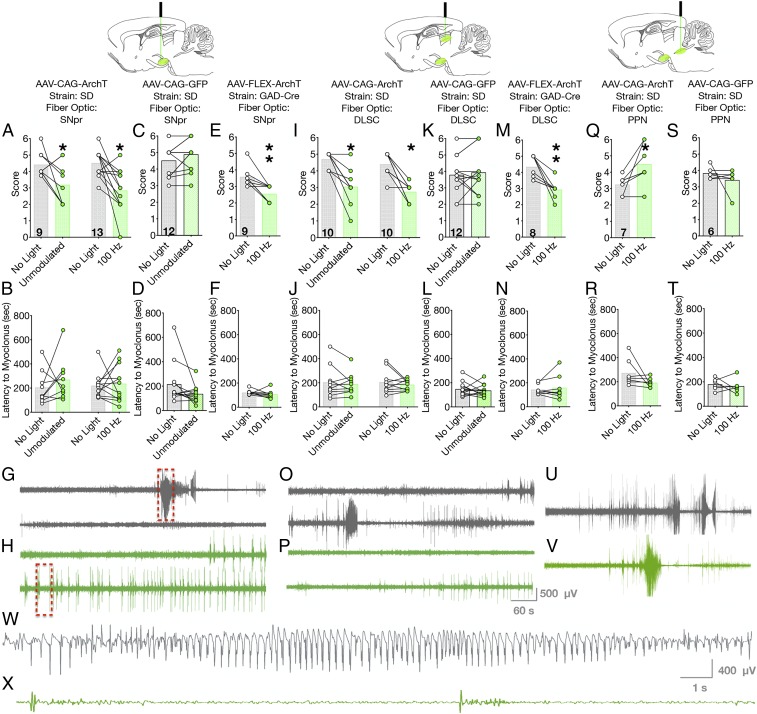
Fig. 2.
Optogenetic silencing of SNpr or nigrotectal terminals reduces PTZ seizures, while optogenetic silencing of nigrotegmental terminals worsens PTZ seizures. Optogenetic silencing of cell bodies within SNpr, with either unmodulated or 100-Hz light delivery, reduces the severity of PTZ-evoked seizures (A) without impacting latency (B). In opsin-negative rats, light delivery was without effect (C and D). Optogenetic inhibition of GABAergic neurons in SNpr suppressed seizure severity (E) without affecting latency (F). Representative electrographic responses from a rat without (G) and with (H) optogenetic inhibition of SNpr. Red boxes indicate expanded view in W and X. (I) Optogenetic silencing of nigrotectal terminals reduced seizure severity, but not (J) latency to seizure. Light delivery to nigrotectal terminals was without effect in opsin-negative rats (K and L). Inhibition of GABAergic nigrotectal terminals reduced seizure severity (M) without affecting latency (N). Antiseizure effects obtained by silencing nigrotectal projections were evident both behaviorally and electrographically (O, without optogenetic silencing; P, with optogenetic silencing). Optogenetic silencing of nigrotegmental terminals increased seizure severity (Q) without effect on latency (R). In opsin-negative rats, light delivery was without effect on seizure severity (S), latency (T), or electrographic activity (U and V). (W and X) Expanded view of the segments indicated by the red boxes in G and H, respectively. Bars indicate means, with individual animal data indicated by circles and lines (*P < 0.05, **P < 0.01).