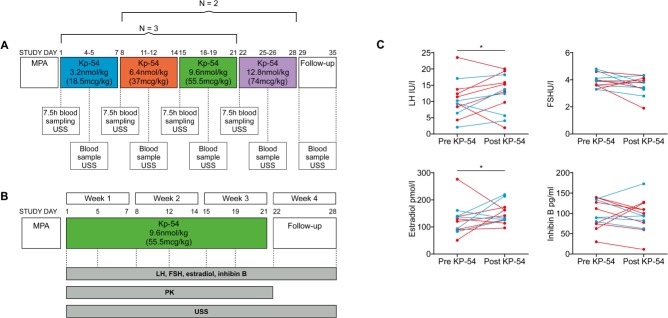
Figure 7.
Schematic of protocol, and gonadotrophin and ovarian hormone responses to KP-54 in 12 individual women with PCOS. In the left panel, a schematic showing the timing of KP-54 administration in the (A) dose-exploration arm and (B) constant dose arm of the study. In both cases, KP-54 was started after a progestogen-induced withdrawal menses and administered s.c. bd for 21 days. Group sizes were N = 5 in (A; split into N = 3 and 2, in two escalation protocols, as depicted) and N = 7 in (B). In (A), multiple blood samples were taken over periods of 7.5 h, as indicated for pharmacokinetic (PK) analysis, and in both, blood samples and transvaginal ultrasound were performed twice weekly as indicated. In the right panels, serum hormone concentrations before and after repeated administration of KP-54 at 3.2, 6.4, 9.6 and 12.8 nmol/kg, and 7 days following the last dose. KP-54 was administered s.c. twice daily for 21 days; Day 1 samples were taken immediately before first dose. Pre- (day-1) and post-KP (7-day after completion of treatments) hormonal levels are shown. Significant overall rises in LH and estradiol (E2) levels during the period of KP-54 administration were detected (Student t tests), with no changes in FSH or inhibin B levels. Blue symbols indicate those patients treated with increasing doses of KP-54, and red symbols denote those treated with a fixed dose of 9.6 nmol/kg twice daily throughout. MPA: medroxyprogesterone acetate, 10 mg bd for 7 days to induce a withdrawal bleed. USS: ultrasound scan.