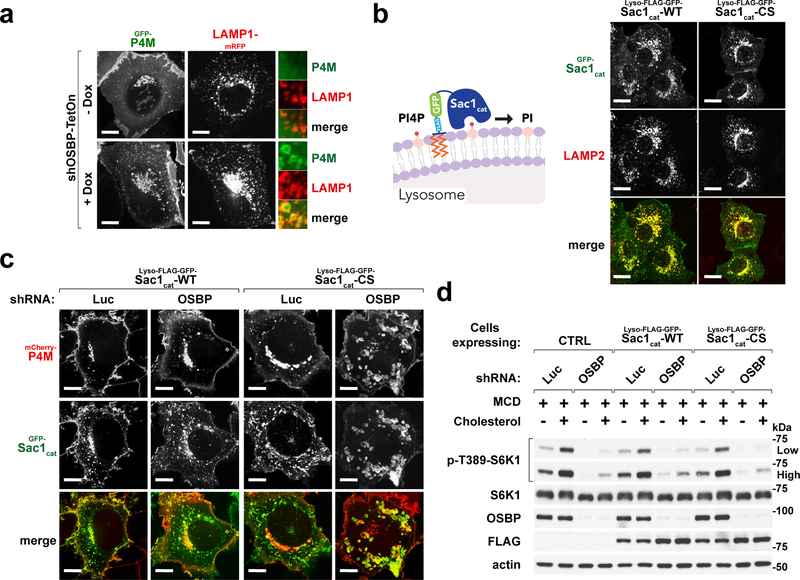
Fig. 5 |. Excess lysosomal PI4P upon OSBP inhibition does not cause mTORC1 inhibition.
a, Depletion of OSBP results in accumulation of PI4P at the lysosomes. HEK-293A cells stably expressing the PI4P-specific probe, GFP-P4M, along with LAMP1-mRFP were depleted of OSBP via doxycycline-induced shRNA. Cells were plated on glass-bottom dishes, allowed to attach overnight and imaged live on a spinning disk confocal microscope. Representative microscopic images are shown. Scale bar, 10 μm. b, Confirmation of lysosomal localization of the lysosome-targeted Sac1 catalytic domain truncations in HEK-293A cells. (left) Schematic diagram showing Sac1 catalytic domain is targeted to the lysosome to hydrolyze its target PI4P. (right) Representative microscopic images are shown. Scale bar, 10 μm. c, Ectopic expression of a lysosome-targeted Sac1 catalytic domain eliminates excess lysosomal PI4P in OSBP-depleted cells. HEK-293T cells co-expressing mCherry-P4M and either catalytically active (WT) or inactive (CS) forms of lyso-Sac1 were depleted of OSBP and subjected to live-cell imaging. Representative confocal microscopic images are shown. Scale bar, 10 μm. d, Lysosomal PI4P buildup caused by OSBP depletion is not responsible for mTORC1 inhibition. Control cells and cells expressing catalytically active and inactive lyso-Sac1 were depleted of OSBP, followed by cholesterol starvation/restimulation, lysis and immunoblotting for the indicated proteins and phospho-proteins. See unprocessed blots in Supplementary Figure 9. Experiments in a–d repeated independently two times.