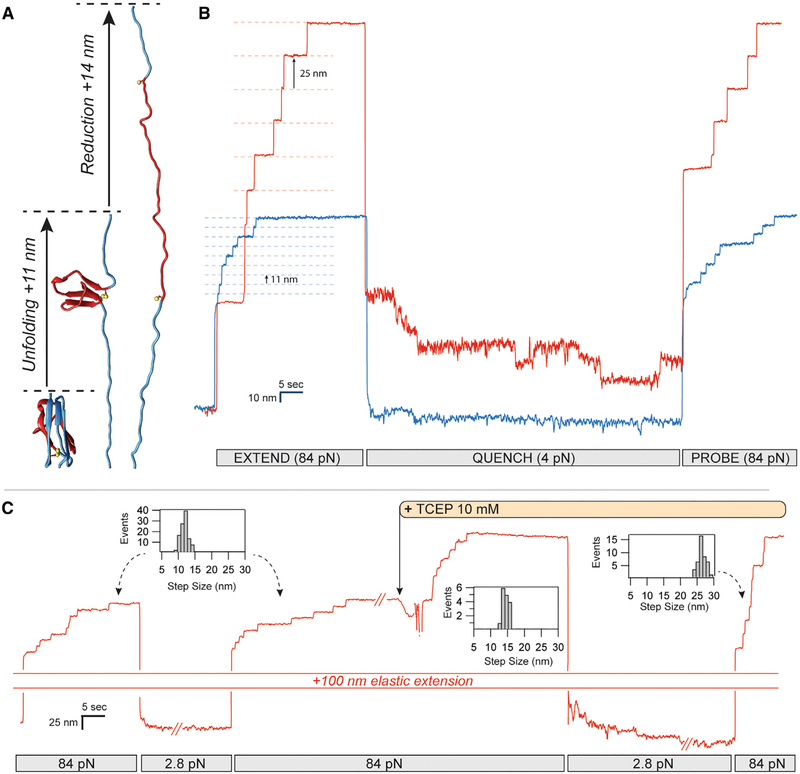
Figure 1. Real-Time Control of Cryptic Disulfide Bonds in Single Polyproteins under Force.
(A) Under force, an Ig domain extends up to its buried disulfide bond, exposing it. Upon reduction, a further extension occurs.
(B) The oxidation status and effect of cryptic disulfide bonds are evident in these two traces of polyproteins containing eight repeats of a titin Ig domain. Oxidized polyproteins extending at a high force of 84 pN increase their length in steps of 11 nm (blue trace), whereas reduced polyproteins extend in steps of 25 nm (red trace). While the rate of folding of the reduced polyprotein at 4 pN is slow, oxidation greatly accelerates the folding rate, driving it to completion at the same force. The increased folding probability at 4 pN is measured from the number of steps recovered with a probe pulse at 84 pN.
(C) The status of a single polyprotein can be easily changed from oxidized to reduced by introducing a reducing agent into the solution (TCEP) while the polyprotein is fully unfolded, exposing its thiols to the solution. Step-size histograms record the changes observed from extending a fully oxidized polyprotein ± 2.1 nm), immediately after the addition of TCEP (14.6 ± 1.6 nm) and after refolding the resulting fully reduced polyprotein (25.6 ± 2.2 nm).