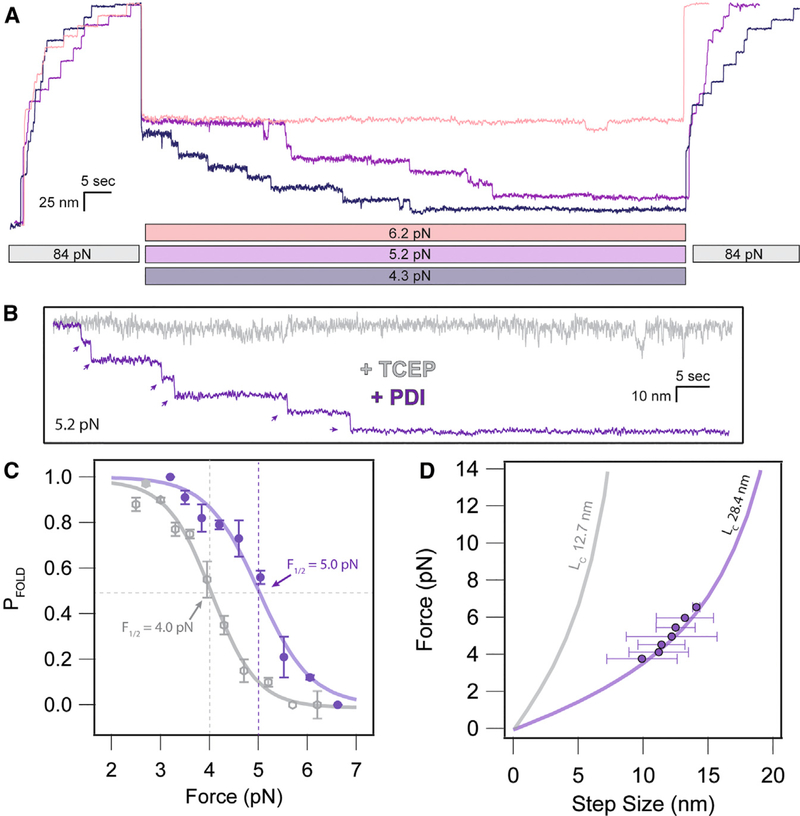
Figure 4. PDI Introduces Disulfide Bonds after Accelerated Polypeptide Collapse.
(A) Folding trajectories of I27 in the presence of 60 mM PDIRED at 6.2, 5.2, and 4.3 pN. The extend and probe pulses contain both 11- and 14-nm steps due to the initial cleavage of the disulfide bond and subsequent oxidoreductase-mediated reformation of the disulfide bond during the quench.
(B) Comparison of individual folding trajectories of I27RED in the presence of 10 mM TCEP versus I27OX after cleavage by 60 μM PDIRED. The greatly favored folded state in the presence of PDI suggests chaperone activity from the oxidoreductase.
(C) The folding probability of I27 is shifted in favor of the folded state in the presence of PDI. The sigmoidal fits demonstrate a 1.0-pN shift of the midpoint folding probability from 4.0 pN in the presence of TCEP to 5.0 pN in the presence of PDIRED.
(D) The folding step sizes observed in the presence of PDI closely followed the FJC model for the I27RED domain, even if there was disulfide refor-mation. This suggests that the oxidoreductase reintroduces the disulfide bond only after there is collapse of the polypeptide backbone.