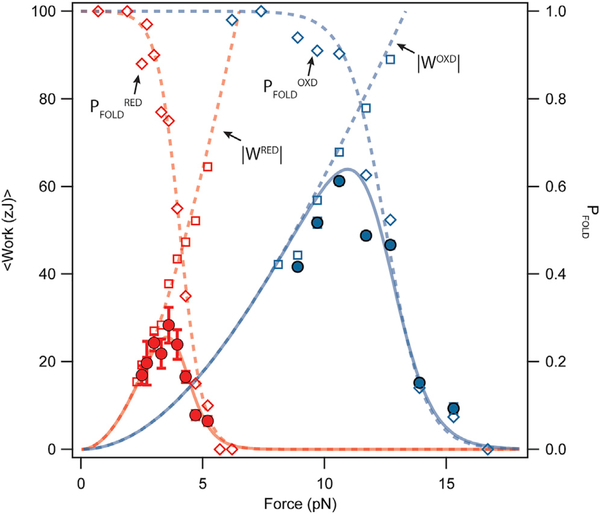
Figure 5. Oxidation Increases the Work Done by Protein Folding and Shifts Its Peak to Higher Forces.
We calculate the expected value of the work delivered by folding steps as the product of folding work times the folding probability. The expected value of the folding work delivered by a reduced Ig domain peaks at a force of 3.5 pN with a value of 25.6 zJ (solid red line), calculated by multiplying the reduced folding probability (empty red squares, dashed line) times the reduced folding work (empty red diamonds, dashed line). Oxidation causes sizeable effects on the mechanical work delivered by protein folding; the expected value of the folding work delivered by an oxidized Ig domain is now 2.5 times larger (63.9 zJ) and peaks at 11 pN (solid blue line); from the oxidized folding probability (empty blue diamonds, dashed line) times the oxidized folding work (empty blue squares, dashed line). However, in both cases, the delivery of the folding work at their peak is very slow due to measured folding kinetics.