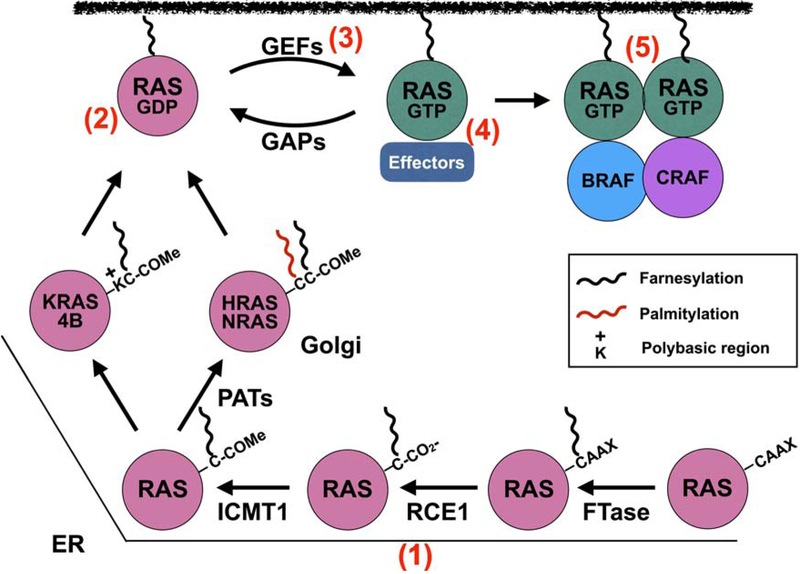
Figure 3. Targeting RAS inhibition.
RAS proteins are initially farnesylated at the ER by farnesyl transferase (FTase) and then HRAS, NRAS, and KRAS4A are translocated to the Golgi where they undergo a second lipidation event mediated by palmitoylacetyl transferases (PATs). KRAS4B possesses a polybasic stretch of Lys residues (K) which function in conjunction with the farnesylated C-term to anchor KRAS4B to the membrane. Many approaches have been utilized to inhibit RAS function from blocking lipidation and membrane association of RAS proteins (e.g., tipifarnib, deltasonamide) (1), interfering with RAS activation by targeting RAS-GDP (e.g., MRTX849, ARS-1620), (2) interaction with GEFs (e.g., DCAI, HBS) (3), interfering with RAS:effector interactions (e.g., ADB7, 3144)(4), and allosteric inhibition of RAS self-association/nanoclustering (e.g. NS1 and K13)(5).