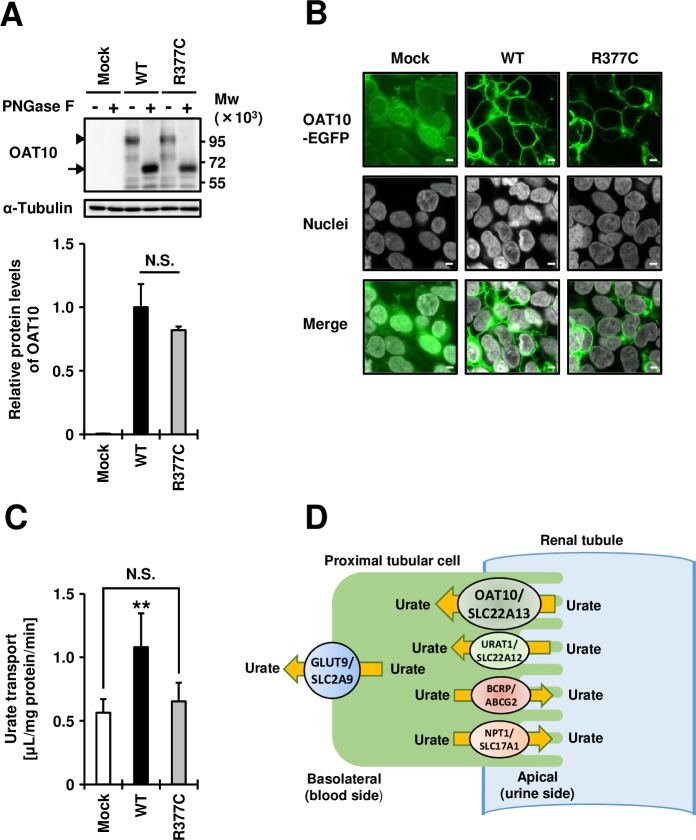
Figure 1.
Effects of Arg377Cys (R377C) on the expression, plasma membrane localisation, and function of the organic anion transporter 10 (OAT10) urate transporter transiently expressed in 293A cells. (A) (Upper) Immunoblot detection of OAT10/SLC22A13 protein in whole cell lysate samples. OAT10 fused with EGFP was detected by an anti-EGFP antibody. Arrowhead, matured OAT10 as a glycoprotein; arrow, non-glycosylated form of OAT10; α-tubulin, a loading control; (Lower) Relative protein levels of OAT10 wild-type (WT) and Arg377Cys (R377C) variant. Data are expressed as the mean±SD, n=3. N.S., not significantly different between groups (two sided t-test). (B) Confocal microscopic observation of cellular localisation. Nuclei were stained with TO-PRO-3 iodide (grey). Bars, 5 µm. (C) Functional analysis. OAT10-expressing 293A cells were incubated with 10 µM of [14C]-urate for 60 s, then the amount of urate incorporated into the cells was measured. Data are expressed as the mean±SD, n=7. **p<0.01 versus the other groups (Tukey-Kramer multiple-comparison test). All experiments were performed 48 hours after plasmid transfection. (D) Proposed physiological model of OAT10 in human kidney. OAT10 is expressed on the apical membrane of renal proximal tubules and mediates reabsorption of urate from urine to blood. Other previously characterised urate reabsorption transporters (URAT1/SLC22A12 and GLUT9/SLC2A9) and urate excretion transporters (BCRP/ABCG2 and NPT1/SLC17A1) are also described.