Figure 4.
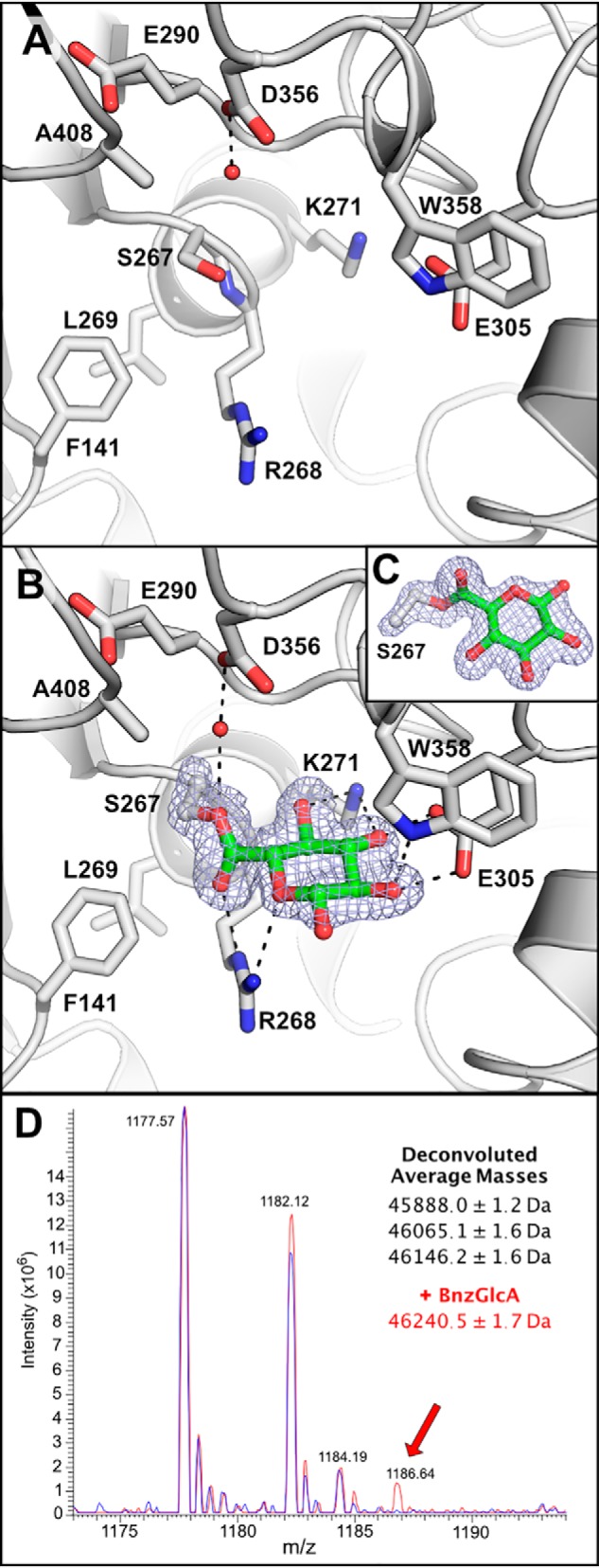
Trapping the glucuronate covalent intermediate in the OtCE15A H408A variant. Shown is the OtCE15A H408A variant in the absence (A; PDB code 6SZ0) and presence of benzyl glucuronoate (B; PDB code 6SZ4). Presumably, the acylation rate was too fast to capture the Michaelis–Menten complex with benzyl glucuronoate over the short crystal soaking period (5 s) but allowed capture of the acyl-enzyme intermediate with the glucuronate moiety covalently linked to the catalytic nucleophile Ser-267. In both structures, a water molecule, hydrogen-bonded to Asp-356, fills the void left from substitution of the catalytic histidine. The covalent serine-glucuronoyl adduct is shown with the density from an omit map, at 4σ, created in Phenix (39) by omitting GlcA and the Cα, Cβ, and Oγ of Ser-267. C, an alternate orientation of the serine-glucuronoyl adduct showing the linkage. D, mass spectrum of the OtCE15A H408A variant in the absence (blue) and presence of benzyl glucuronate (red) leads to the production of a new mass consistent with the glucuronate covalent intermediate.
