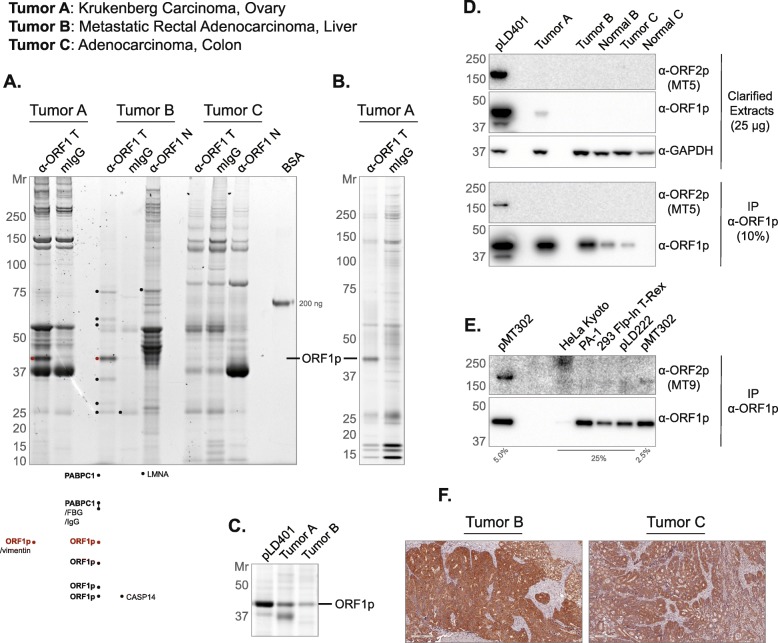
Fig. 4.
Protein Staining and Western Blotting of anti-ORF1p IPs and extracts. a Three tumors (labeled TOP, LEFT) were used as starting material for ORF1p affinity isolations (α-ORF1p T), including mock-capture controls using mouse IgG affinity medium with tumor extracts (mIgG), and matched normal tissue with anti-ORF1p affinity medium (α-ORF1p N). The eluted material was electrophoresed (4–12% Bis-Tris NuPAGE) and Coomassie G-250 stained [53]; a 200 ng BSA standard is displayed as a staining intensity gauge. Each lane contains a 200 mg-scale isolation using 10 μl of affinity medium. Several bands were cut and analyzed by LC-MS/MS - the highest-ranking proteins are listed (see Methods) (b) Tumor A anti-ORF1p affinity capture was repeated using a slightly modified procedure (see Methods). 30% of 100 mg-scale affinity isolations using 15 μl of affinity medium have been electrophoresed and Sypro Ruby stained. c Comparison of ORF1p yield from anti-ORF1p affinity isolations. pLD401 is a codon-optimized L1 sequence (OrfeusHs), ectopically expressed in HEK-293TLD [10]. Here, 80% of 100 mg-scale affinity isolations using 10 μl of affinity medium have been electrophoresed and Coomassie G-250 stained. d Western blotting of the same materials used in (c), including Tumor C and matched normal tissues. Here, 25 μg of the whole cell extract have been probed for ORF2p, ORF1p, and GAPDH as a control. 10% of α-ORF1p affinity isolates have also been probed for ORF2p and ORF1p. e A collection of cell lines were assessed by anti-ORF1p affinity capture. pMT302 is derived from a naturally occurring L1 sequence (L1RP), ectopically expressed in HEK-293TLD [10]. pLD222 is a plasmid harboring a doxycycline-inducible GFP construct ectopically expressed in HEK-293TLD; here included as a control for pMT302. f IHC using α-ORF1p on (LEFT) Tumor B and (RIGHT) Tumor C. α-ORF2p clones MT5 (panel D) and MT9 (panel E) are described in this study (see Figs. 2 and 3)