The severity and effects of damage to short- and long-range structural connections in multiple sclerosis are unknown. Meijer et al. report that long-range connections are more severely damaged than short-range connections. Damage to long-range connections is more strongly associated with reduced structural network efficiency, abnormal functional connectivity, and impaired cognition.
Keywords: multiple sclerosis, MRI, structural brain network, functional brain network, cognition
Abstract
An efficient network such as the human brain features a combination of global integration of information, driven by long-range connections, and local processing involving short-range connections. Whether these connections are equally damaged in multiple sclerosis is unknown, as is their relevance for cognitive impairment and brain function. Therefore, we cross-sectionally investigated the association between damage to short- and long-range connections with structural network efficiency, the functional connectome and cognition. From the Amsterdam multiple sclerosis cohort, 133 patients (age = 54.2 ± 9.6) with long-standing multiple sclerosis and 48 healthy controls (age = 50.8 ± 7.0) with neuropsychological testing and MRI were included. Structural connectivity was estimated from diffusion tensor images using probabilistic tractography (MRtrix 3.0) between pairs of brain regions. Structural connections were divided into short- (length < quartile 1) and long-range (length > quartile 3) connections, based on the mean distribution of tract lengths in healthy controls. To determine the severity of damage within these connections, (i) fractional anisotropy as a measure for integrity; (ii) total number of fibres; and (iii) percentage of tract affected by lesions were computed for each connecting tract and averaged for short- and long-range connections separately. To investigate the impact of damage in these connections for structural network efficiency, global efficiency was computed. Additionally, resting-state functional connectivity was computed between each pair of brain regions, after artefact removal with FMRIB’s ICA-based X-noiseifier. The functional connectivity similarity index was computed by correlating individual functional connectivity matrices with an average healthy control connectivity matrix. Our results showed that the structural network had a reduced efficiency and integrity in multiple sclerosis relative to healthy controls (both P < 0.05). The long-range connections showed the largest reduction in fractional anisotropy (z = −1.03, P < 0.001) and total number of fibres (z = −0.44, P < 0.01), whereas in the short-range connections only fractional anisotropy was affected (z = −0.34, P = 0.03). Long-range connections also demonstrated a higher percentage of tract affected by lesions than short-range connections, independent of tract length (P < 0.001). Damage to long-range connections was more strongly related to structural network efficiency and cognition (fractional anisotropy: r = 0.329 and r = 0.447. number of fibres r = 0.321 and r = 0.278. and percentage of lesions: r = −0.219; r = −0.426, respectively) than damage to short-range connections. Only damage to long-distance connections correlated with a more abnormal functional network (fractional anisotropy: r = 0.226). Our findings indicate that long-range connections are more severely affected by multiple sclerosis-specific damage than short-range connections. Moreover compared to short-range connections, damage to long-range connections better explains network efficiency and cognition.
Introduction
Multiple sclerosis is a chronic demyelinating and neurodegenerative disease of the CNS (Stys et al., 2012). Although the clinical course of individuals with multiple sclerosis is characterized by a wide variety of symptoms, up to 70% of them experience some degree of cognitive impairment (Chiaravalloti and DeLuca, 2008). Optimal cognitive function is thought to depend on a delicate balance between local and global signal processing, which is made possible by the topological configuration of white matter tracts between grey matter regions (Bressler and Menon, 2010; Park and Friston, 2013). The structural brain network is topologically arranged such that the spatial embedding of the wiring is cost-efficient, combining high efficiency with low connection cost (van den Heuvel and Sporns, 2013), i.e. a small-world network (Bassett and Bullmore, 2006). Despite favouring short-range, low-cost connections, the network also contains a small proportion of more ‘expensive’ long-range connections (Laughlin and Sejnowski, 2003). The latter are especially relevant for integrating information between different parts of the brain (Park and Friston, 2013).
In multiple sclerosis, the structural backbone of the brain is damaged by white matter lesions (Lassmann et al., 2007) and normal-appearing tissue integrity loss (Evangelou et al., 2000; Kutzelnigg et al., 2005; Dineen et al., 2009; Yu et al., 2012; Meijer et al., 2016). These pathological perturbations are rarely confined to a single region and are likely to change the information flow throughout the structural network (Fornito et al., 2015). A minor shift in the balance between short- and long-range connections is likely to change the efficiency of the structural network (van den Heuvel and Sporns, 2013). In addition, it is hypothesized that short- and long-range connections could have a different role in establishing brain function, which might indicate that damage to either one could have different effects (Zeki and Shipp, 1988; Passingham et al., 2002; Gallos et al., 2012). It is expected that the functionality of the costly long-range connection might outweigh their cost, and that their demise thus might have larger consequences (van den Heuvel et al., 2012). It is, however, not known whether short- and long-range connections are equally damaged in multiple sclerosis or whether one is more vulnerable to the pathology than the other. Also it is unknown how damage to either short- or long-range connections may influence brain function and especially cognitive performance. It might be that damage to long-range connections could better explain the cognitive status of patients with multiple sclerosis than pathological changes in the short-range counterparts, because of the crucial integrative role of long-range connections (Park and Friston, 2013).
In this study, we therefore investigated the severity of structural damage in short- and long-range connections and its impact on the efficiency of the global structural brain network. Furthermore, we investigated whether damage in short or long-range connections could best explain functional resting-state changes and cognitive dysfunction.
Materials and methods
Participants
For this retrospective study, 133 patients with long-standing multiple sclerosis [44% males, age 50.82 ± 7.04 years, symptom duration 20.66 years (interquartile range: 16.33–23.99)] were included, as well as 48 healthy controls (38% male, age 54.21 ± 9.59 years), all part of the larger Amsterdam multiple sclerosis cohort (Steenwijk et al., 2014; Daams et al., 2015). All patients were diagnosed with clinically definite multiple sclerosis according to the 2010 revised McDonald criteria, and had a relapse-free period with no steroid treatment for at least 2 months prior to participation. Disease-modifying treatments included β-interferons (n = 23), glatiramer acetate (n = 7), natalizumab (n = 9), or other immunosuppressive therapy (n = 6). Approval for this study was obtained from the institutional ethics review board of the Amsterdam University Medical Center, location VU Medical Center, and subjects had given written informed consent prior to participation. The cohort has been described in previous publications, but none of the studies investigated structural network properties (Steenwijk et al., 2015; Eijlers et al., 2018; Meijer et al., 2018).
Neuropsychological and clinical evaluation
On the day of MRI, all participants underwent extensive neuropsychological testing based on the expanded version of the Brief Repeatable Battery of Neuropsychological tests, as previously described (Meijer et al., 2017). The assessed cognitive tests were: (i) the symbol digit modalities test (information processing speed); (ii) selective reminding test (verbal memory); (iii) memory comparison test (working memory); (iv) Stroop colour-word test (attention); (v) concept shifting task (executive functioning); and (vi) spatial recall test (visuospatial memory). These cognitive scores were converted to z-scores relative to the mean and standard deviation (SD) of healthy controls. Next, these scores were averaged to calculate an average cognitive performance composite score. Additionally, the Expanded Disability Severity Status (EDSS) was conducted to assess overall disability.
MRI
MRI was performed on a 3 T scanner (GE Signa HDxt) using an eight-channel phased-array head-coil. The imaging protocol included a 3D T1-weighted inversion-prepared fast spoiled gradient recall sequence (FSPGR, repetition time 7.8 ms, echo time 3 ms, inversion time 450 ms, flip angle 12°, sagittal 1.0 mm sections, 0.94 × 0.94 mm2 in-plane resolution) for volumetric measurements, a 3D fluid-attenuated inversion-recovery sequence (FLAIR, repetition time 8000 ms, echo time 125 ms, inversion time 2350 ms, sagittal 1.2 mm slices, 0.98 × 0.98mm2 in-plane resolution) for lesion detection and a diffusion tensor imaging (DTI) sequence covering the entire brain using five volumes without directional weighting (i.e. b = 0 s/mm2) and 30 volumes with non-collinear diffusion gradients [echo planar imaging (EPI), b = 1000 s/mm2, repetition time 13 000 ms, echo time 91 ms, flip angle 90°, 2.4 mm contiguous axial slices, 2 × 2mm2 in-plane resolution]. Brain function was assessed using resting-state functional MRI with whole-brain coverage using 202 volumes, of which the first two were discarded (EPI, repetition time 2200 ms, echo time 35 ms, flip angle 20°, 3 mm contiguous axial slices, 3.3 × 3.3 mm2 in-plane resolution).
Volumetric measures
To reduce the impact of lesions on registration algorithms and volumetric measures, hyperintense lesions were automatically segmented on FLAIR images and filled on the 3D T1 using LEAP (Chard et al., 2010; Steenwijk et al., 2013). Based on these segmentations, the total lesion load was computed for each patient. SIENAX (part of FSL 5) was used to calculate grey matter volume and white matter volume and to normalize these measures for head size based on v-scaling. Normalized cortical volumes were computed by subtracting deep grey matter segmentations derived with FSL-FIRST from the SIENAX-based grey matter segmentation.
Structural connectivity
Eddy current distortion correction was done using the FMRIB Diffusion Toolbox (FDT; also part of FSL). Probabilistic tractography using the fibre orientation distribution (FOD) was then performed by using MRtrix 3.0 (Tournier et al., 2012). This model reconstructs N streamlines by randomly putting seeds in white matter and estimates the local FOD using constrained spherical deconvolution (Tournier et al., 2007). To adjust to the 30 non-collinear diffusion directions available in our data, we followed the developer recommendation to restrict the maximum spherical harmonic order (lmax) to 6. Whole-brain probabilistic tractography was then performed by randomly seeding 100 million fibres within the brain mask for each subject.
To be able to create structural networks, cortical grey matter nodes were defined by processing the 3D T1-weighted image of each subject with the FreeSurfer 5.3 pipeline. The automated anatomical labelling (AAL) atlas was used to define cortical nodes on the cortical surface (Tewarie et al., 2014). As mentioned above, deep grey matter nodes were derived by processing of 3D T1-weighted images using FIRST. The cortical and deep grey matter regions were transformed to DTI space using FLIRT (also part of FSL), using the previously obtained T1 to DTI rigid transformation matrix and nearest-neighbour interpolation.
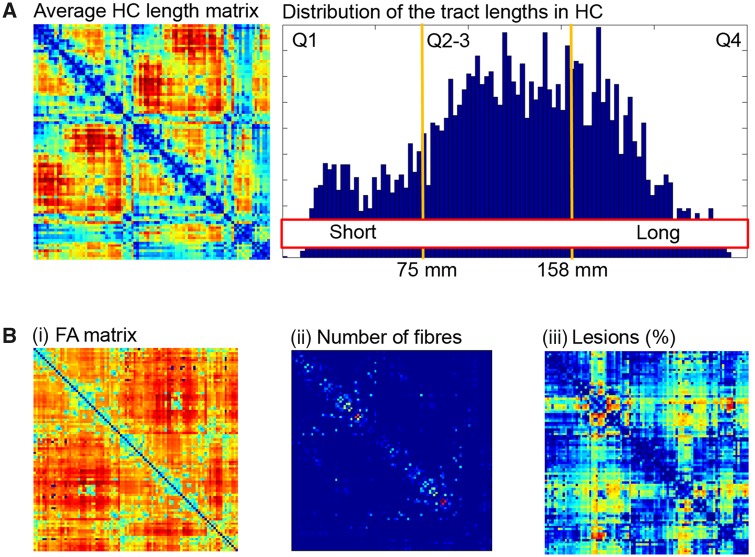
To be able to categorize the connecting tracts into short- and long-range connections, the averaged fibre length for each connecting tract was determined. Structural connections were divided into short (<Q1), middle (Q2–Q3) and long (>Q3), based on the histogram of tract lengths in healthy controls (Fig. 1A). For each connecting pair of nodes different outcome measures were extracted that could reflect the level of structural damage, namely (i) the average fractional anisotropy as a measure of integrity; (ii) the number of fibres; and (iii) the average percentage of tract affected by white matter lesions (Fig. 1B). The average percentage of the tract affected by white matter lesions was calculated by sampling the overlap between an individual binary lesion map and the fibres constructed by MRtrix, this value was normalized for the length of the tract. The three structural damage measures were averaged over all short- and long-range connections to determine the level of structural damage within each type of connection. To determine the severity of damage, fractional anisotropy and number of fibres observed in short- and long-range connections were converted to z-scores based on the mean and standard deviation observed in the healthy controls. This normalization step also assured that normal variations in these outcome measures between short- and long-range connections were reduced (i.e. shorter connections typically have higher fractional anisotropy and a higher total number of connections than long connections).
Figure 1.
Determining the degree of damage in short- and long-range white matter tracts. (A) Based on the distribution of the tract lengths in healthy controls (HC), structural connections were categorized into short-range (<75 mm) and long-range connections (>158 mm). (B) For these short-and long-range connections three different measures that reflect the severity of structural damage were extracted, namely (i) fractional anisotropy (FA); (ii) total number of fibres; and (iii) percentage of tract affected by lesions (case example).
Structural network efficiency
The efficiency of each individual structural network, consisting of regions as nodes and the number of fibres as edges, was computed using the Brain Connectivity Toolbox (Rubinov and Sporns, 2010). To increase the robustness of the determined structural networks, the strongest 20% connections were selected and binarized (Achard and Bullmore, 2007; Bullmore and Sporns, 2012). After that, the global efficiency was computed based on these binary structural networks to determine the extent to which information is efficiently distributed and globally integrated in the network.
Functional connectivity
Preprocessing of functional MRI data was performed using the FMRIB Software Library (part of FSL 5). The MELODIC default pipeline consisted of motion correction, removal of non-brain tissue, spatial smoothing using a 5-mm full-width at half-maximum Gaussian kernel and high-pass temporal filtering to cut off frequencies <0.01 Hz. In addition, to denoise functional MRI data further, and to reduce motion, physiological and other artefacts, ICA-based X-noisefier (ICA-FIX) was applied as trained for application to a comparable standard functional MRI dataset (Salimi-Khorshidi et al., 2014). To remove signal originating from residual non-brain tissue as well as from voxels sensitive to EPI distortions, voxels with a time-averaged signal intensity in the lowest quartile of the robust range were excluded (Eijlers et al., 2017; Meijer et al., 2017).
For each subject, functional connectivity matrices were constructed in which cortical nodes were defined by the AAL atlas, deep grey matter nodes were defined by FIRST segmentations and the strength of edges was measured by Pearson correlations between the average time series at the nodes. In short, the AAL atlas was registered to native 3D T1 space with inverted non-linear registration parameters, using nearest neighbour interpolation. The atlas was then multiplied with the SIENAX-derived grey matter segmentations to include grey matter only. Deep grey matter regions derived by FIRST were appended to the AAL atlas in native 3D T1 space to create an atlas that covers both cortical and subcortical regions. Subsequently, boundary-based registration was used to derive the transformation between functional MRI space and T1-weighted space. The inverted matrices were used to register the final atlas to each individual functional MRI scan using nearest neighbour interpolation.
Functional connectivity matrices were then constructed by calculating Pearson correlations between the averaged time series of all these pairs of nodes. Each individual connectivity matrix was transformed to a vector and was correlated with the values of an average healthy control matrix. The latter was created based on all healthy controls (n = 96) from the Amsterdam multiple sclerosis cohort (Meijer et al., 2018). The correlation coefficient between the individual and the average healthy control matrix reflects the functional connectivity similarity index. A high value (i.e. strong correlation coefficient) indicates that the individual functional connectivity matrix corresponds well with the average healthy control matrix, whereas a lower value indicates that the functional connectivity is not similar to the average healthy control matrix.
Statistical analyses
Statistical analyses of the demographic, clinical and whole-brain MRI variables were performed in SPSS version 22 (Armonk, NY, USA). All demographic, clinical and volumetric MRI variables were checked for normality using the Kolmogorov-Smirnov test and histogram inspection. Non-parametric tests were used to compare not normally distributed measures of interest. Group differences for normally distributed demographic variables were assessed by multivariate general linear model analyses, with age and sex as covariates. These models were also used to compare the level of structural damage (i.e. fractional anisotropy-based integrity, number of fibres and percentage of lesions) in short-and long-range connections between patients with multiple sclerosis and healthy controls. In addition, paired t-tests were conducted to investigate whether long-range connections were more severely damaged than short-range connections in the patient group. Measures of structural damage within both types of connections were correlated to structural network efficiency, functional connectivity similarity index and cognition (i.e. averaged cognitive performance and symbol digit modalities scores). For group comparisons and correlations, P-values of <0.05 were considered statistically significant after false discovery rate (FDR) correction for multiple comparisons; corrected P-values are reported.
Data availability
Anonymized data, not published in the article, will be shared on reasonable request from a qualified investigator.
Results
Demographics, clinical and cognitive characteristics
Demographic and clinical characteristics are shown in Table 1. Sex and level of education did not differ between healthy controls and people with multiple sclerosis, but the multiple sclerosis group was younger than the healthy control group (F = 4.99; P = 0.03). The EDSS indicated a moderately affected multiple sclerosis population with an on average disease duration of 20 years. Average cognitive performance was lower in patients with multiple sclerosis than healthy controls (F = 32.41; P < 0.001; z-score = −0.95).
Table 1.
Demographics, clinical and cognitive characteristics
| Healthy control group | Multiple sclerosis group | |
|---|---|---|
| n | 48 | 133 |
| Age (SD) | 54.2 (9.6) | 50.8 (7.0)# |
| Sex, male/female | 18/30 | 59/74 |
| Education (IQR)* | 5 (3–7) | 6 (3–7) |
| Multiple sclerosis type, RR/SP/PP | – | 84/32/17 |
| EDSS (IQR)* | – | 3.5 (3.5–5.5) |
| Disease duration (IQR)* | – | 20.7 (16.3–24.0) |
For normally distributed variables, the mean and standard deviation (SD) are provided, while the median and interquartile range (IQR) are provided for non-normally distributed variables (*). #Significant difference between patients with multiple sclerosis and healthy controls (P < 0.05; FDR-corrected). EDSS = expanded disease severity scale; PP = primary progressive; RR = relapsing remitting; SP = secondary progressive.
Table 2.
Global structural MRI measures
| Healthy control group | Multiple sclerosis group | |
|---|---|---|
| Volumetric structural measures | ||
| Total brain volume, l | 1.50 (0.05) | 1.43 (0.08)# |
| White matter volume, l | 0.70 (0.03) | 0.67 (0.04)# |
| Grey matter volume, l | 0.80 (0.04) | 0.77 (0.05)# |
| Deep grey matter volume, ml | 61.57 (3.14) | 54.63 (6.96)# |
| Total lesion volume, ml* | 12.77 (7.14–21.33)# | |
| Global structural network measures | ||
| Structural network efficiency | 0.543 (0.005) | 0.541 (0.005)# |
| Average fibre length | 125.33 (121.06–129.45) | 124.10 (120.38–127.19) |
| DTI-based fractional anisotropy | 0.40 (0.02) | 0.38 (0.02)# |
| Number of fibres | 7467.00 (603.65) | 7348.51 (659.27) |
For normally distributed variables, the mean and standard deviation (SD) were provided, while the median and interquartile range are provided for non-normally distributed variables (asterisk). All volumetric measures were normalized for head size. #Significant difference between multiple sclerosis and healthy controls (P < 0.05; FDR-corrected).
Table 3.
Correlation coefficients between the extent of structural damage and structural network efficiency, cognition and the functional network
| Structural network efficiency | Cognitive function | FC similarity index | |
|---|---|---|---|
| Short-range | |||
| FA-based integrity | n.s. | 0.180 | n.s. |
| Number of fibres | n.s. | 0.229 | n.s. |
| Percentage of lesions | −0.218 | −0.441 | n.s. |
| Long-range | |||
| FA-based integrity | 0.329 | 0.447 | 0.226 |
| Number of fibres | 0.321 | 0.278 | n.s. |
| Percentage of lesions | −0.219 | −0.426 | n.s. |
FA = fractional anisotropy; FC = functional connectivity; n.s. = non-significant.
Global structural MRI measures
For all global volumetric measures, significant differences were observed between healthy controls and patients with multiple sclerosis (P < 0.001), indicating lower volumes in patients with multiple sclerosis. For the structural network measures, reduced structural network efficiency and reduced fractional anisotropy of the connections within the entire structural network were observed in patients with multiple sclerosis (F = 4.73; P = 0.046; F = 19.70; P < 0.001, respectively). Average whole-brain fibre length and number of fibres were not different between groups.
Damage to short- and long-range connections
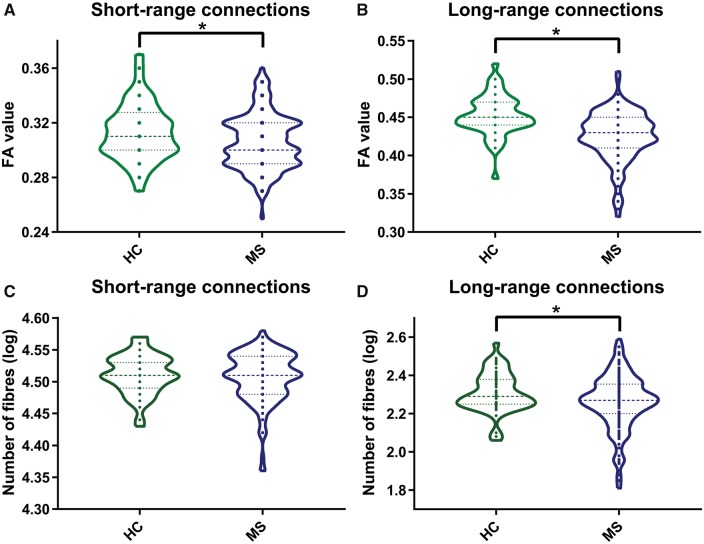
In general, long-range connections had a higher fractional anisotropy (T = 75.95; P < 0.001) and a lower number of fibres (T = 163.62; P < 0.001) than short-range connections (Fig. 2). Mean fractional anisotropy (FA) was reduced in short and long white matter connections in patients with multiple sclerosis compared to healthy controls (Fig. 2A and B). Long-range connections showed the largest reduction in white matter integrity (z-scoreFA = −1.03, F = 28.89; P = 0.002), whereas short-range connections were less severely affected (z-scoreFA = −0.34, F = 4.67; P < 0.05) relative to healthy controls. In addition, within-subject analyses demonstrated that this difference in severity of white matter integrity loss between short- and long-range connections was significant (T = 8.36; P = 0.002; Fig. 3A).
Figure 2.
Fractional anisotropy and number of fibres within short- and long-range connections. Lower fractional anisotropy (FA) values were observed in (A) short-range (P = 0.03) and (B) long-range connections (P < 0.001) in multiple sclerosis patients (MS) relative to healthy controls (HC). The number of fibres was only reduced in (C) long-range connections (P = 0.001) in multiple sclerosis patients relative to healthy controls. In the violin plots the median and interquartile interval are represented as dashed lines. Arms indicate a significant difference (*P < 0.05, FDR-corrected).
Figure 3.
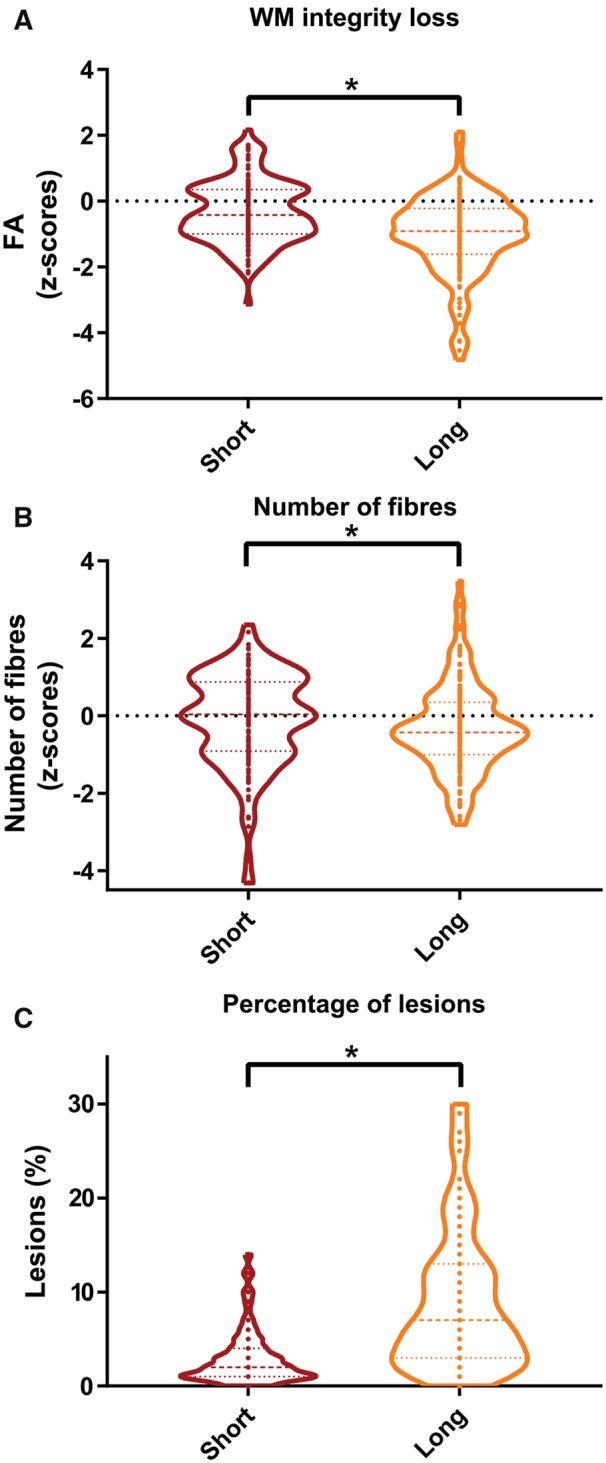
Severity of structural damage in short- and long-range connections. (A) Loss of white matter (WM) integrity as measured by fractional anisotropy, (B) loss of the number of fibres and (C) the percentage of lesions were all more severely affected in long-range than short-range connections (all P < 0.001). In the violin plots the median and interquartile interval are represented as dashed lines. Arms indicate a significant difference (*P < 0.05, FDR-corrected).
The number of connections (i.e. fibre count) was only reduced for the long white matter connections in patients with multiple sclerosis compared to healthy controls (z-scorecount = −0.44, F = 9.34; P < 0.01; Fig. 2C and D). Within-subject analyses demonstrated that the number of connections was more severely reduced in long-connections than in short-range connections (T = 2.08; P < 0.05; Fig. 3B). In addition, long-range connections showed a higher percentage of tract affected by lesions than short-range connections (T = −14.96. P = 0.002; Fig. 3C). To examine whether the extent of damage was gradual or not, the level of damage was also computed for the category in between the short- and long-range connections, namely the middle-range connections (Supplementary Table 1). The values for fractional anisotropy and percentage of tract affected by lesions for the middle-range connections were approximately inbetween the short- and long-range values, suggesting a linear relationship. For the number of fibres the relation seemed to be more non-linear or exponential.
Impact on structural network efficiency
In patients with multiple sclerosis, all damage-related measures of long-range connections were associated with structural network efficiency. Positive correlations were observed between structural network efficiency and fractional anisotropy (r = 0.329, P < 0.01) and number of fibres (r = 0.321, P < 0.01) of long-range connections. The lesional fractions of short- and long-range connections were correlated with structural network efficiency, indicating that increasing lesion load within a tract was associated with lower structural network efficiency values (rho = −0.218, P = 0.02 and rho = −0.219, P = 0.02, respectively).
Impact on the functional brain network
The functional connectivity similarity index was lower in patients with multiple sclerosis than in the healthy controls (F = 11.75; P < 0.001). Of the structural connectivity measures, fractional anisotropy of long-range connections was related to the functional connectivity similarity index (rho = 0.226, P < 0.05) indicating that more severe damage in long-range connections was related to a more abnormal functional connectivity similarity index. No correlation was observed between the fraction of lesions and functional connectivity.
Impact on cognitive performance
Only the fractional anisotropy of long-range connections was correlated to symbol-digit modality scores (r = 0.388, P = 0.002). The number of fibres of long-range connections was more strongly related to these cognitive scores than the number of fibres of short-range connections (r = 0.257, P < 0.01 versus r = 0.200, P = 0.03, respectively). Again, the strongest correlations were observed for the percentage of lesions within short- and long-range connections (rho = −0.402, P = 0.002 and rho = −0.369, P = 0.002, respectively).
Fractional anisotropy of long-range connections was more strongly related to average cognitive performance than the integrity of short-range connections (r = 0.447, P = 0.002 versus r = 0.180, P = 0.04, respectively). The correlations between the number of fibres in short- and long-range connections and averaged cognition were significant, but comparable (r = 0.229, P = 0.01 versus r = 0.278, P = 0.002). The strongest correlations with cognition were observed for the percentage of lesions within short- and long-range connections (rho = −0.441, P = 0.002 and rho = −0.426, P = 0.002, respectively).
To investigate the best predictors of cognitive performance in patients with multiple sclerosis, a backward regression model was conducted including the structural damage measures, age, sex and education. The final model (R2 = 0.41; P<0.001) contained the following predictors for worse cognitive performance: higher percentage of tract affected by white matter lesions in long-range connections (standardized β = −0.375; P<0.001), lower educational level (standardized β = 0.244; P<0.01), lower fractional anisotropy of long-range connections (β = 0.231; P = 0.01) and fewer fibres in short-range connections (standardized β = 0.213; P = 0.002).
Discussion
The results of this study suggest that long-range connections are more severely damaged by multiple sclerosis pathology. This is indicated by a more severe loss of tract integrity (i.e. reduced fractional anisotropy), reduced number of tractography-based fibres and a higher percentage of tracts affected by lesions in long-range connections, relative to their short-range counterparts. Moreover, compared to short-range connections, damage to long-range connections was more strongly associated with structural network efficiency, the functional network and cognition. Our results together emphasize the importance of an optimal large-scale brain network organization, consisting of many intact short-range connections and a small number of efficient connections spanning long distances, to maintain structural network efficiency, normal functional network organization and cognition in patients with multiple sclerosis.
Pathology in long-range connections: not simply a matter of probability
In general, the connections of the structural network of patients with multiple sclerosis showed reduced integrity compared to healthy controls, while the average number of fibres and the average length of the white matter tracts did not differ between the two groups. After categorizing the white matter tracts into different types of connections according to their length, more severe damage was observed in long-range connections. This might indicate that multiple sclerosis pathology especially interrupts structural pathways connecting remote brain regions in different parts of the brain. It could be that the stereotypical distribution of white matter lesions with a predominantly periventricular pattern (Kolasinski et al., 2012; Haider et al., 2016) explains why long-range connections are more severely damaged. The majority of the long-range connections are located close to the ventricles (e.g. interhemispheric connections), which could explain the observed higher percentage of lesions in long-range connections than in short-range connections. Mechanisms that could explain the more frequent occurrence of white matter lesions around the ventricle than elsewhere, have not been determined yet. Nevertheless, lesions do usually occur around subependymal veins (Adams et al., 1987) and perivascular inflammatory infiltrates are seen in multiple sclerosis lesions and normal appearing white matter (Hauser et al., 1986; Kutzelnigg et al., 2005). It is hypothesized that all kinds of CSF-mediated factors and inflammatory immune cells can enter the brain from this site and thus could affect the white matter of periventricular long-range connections predominantly. Of note, another explanation would be that long-range connections cover a larger area of the brain than short-range connection and therefore one could assume that a long-range connection is statistically more likely to get damaged. The measure regarding the percentage of tract affected by lesions, however, showed a clear difference with respect to the level of damage between short-and long-range connections while taking the length of the tract into account, which makes it less likely that long-range connections are more severely affected simply because they are covering a larger area of brain. From a microscopic point of view, further research is needed to investigate whether long-range connections are more severely damaged by multiple sclerosis pathology because of their intrinsic biochemical make-up. It could be hypothesized, for example, that the higher metabolic level of long-range connections makes them more vulnerable to oxidative stress and therefore to energy failure under stress (Perge et al., 2012; Bercury and Macklin, 2015).
Shift in the network balance and loss of structural network efficiency
Damage to long-range connections was predominantly related to a less efficient network organization in our cohort of multiple sclerosis patients. The more severe damage in these tracts, the larger the loss in structural network efficiency, suggesting a less optimal balance between a structurally segregated and integrative organization. Most likely because damage to long-range white matter tracts is thought to disrupt the brain’s small-world architecture (Sharp et al., 2014) and could shift the network balance towards the shorter connections. Long-distance connections could be seen as topological short-cuts to reduce the informational distance between brain areas, thereby facilitating rapid and efficient communication (Sporns and Zwi, 2004; Bassett and Bullmore, 2017). With increasing levels of damage, long-range connections are no longer able to facilitate long-distance communication and thus more steps via short-range connections are needed to transfer information from one part of the brain to another, making the network less efficient. Previous studies have already shown that the efficiency and small-world features of the structural brain network are sensitive to pathology in patients with multiple sclerosis (He et al., 2009; Shu et al., 2011; Charalambous et al., 2019). An increasing lesion load is associated with a less prominent small-world pattern and a less efficient network (He et al., 2009). Also the efficiency of the motor network is reduced in multiple sclerosis, which was a stronger predictor than conventional MRI measures for clinical status (Pardini et al., 2015). However, this study importantly elaborates on these findings by showing that network efficiency in patients with multiple sclerosis is especially sensitive for damage to long-range connections.
Relevance of long-range connections for cognition and brain function
It was also observed that damage in long-range connections (i.e. reduced white matter integrity) was more strongly associated with functional brain network than damage in short-range connections. These functional changes could arise from disrupted neuronal conduction due to damage to white matter tracts. Previous studies have already suggested that long-range connections may play an especially important role for the organization of functional brain modules (Gallos et al., 2012). Our results might indicate that intact long-range connections are needed to maintain a functional network similar to that of healthy subjects. Furthermore, our findings indicate that cognitive impairment could be related to which extent long-range connections are damaged. A higher percentage of tracts affected by white matter lesions in long-range connections was the strongest predictor for cognitive dysfunction. This supports the hypothesis that especially long-range connections entail global integration and thus facilitate higher cognitive processes. Local integration entails specialized functional processing mediated by short-range connections, intrinsic to a module at any scale, whereas global integration subserves higher cognition, facilitated by long-range connections, such as extrinsic cortico-cortical connections (Bressler and Menon, 2010). The stronger association between damage to long-range connections and structural network efficiency, functional network and cognition could also be explained by the fact that these longer connections are in the minority and therefore less redundant than short-range connections, which might explain the larger impact of connections that span larger distance (Tononi et al., 1999). In addition to the explanations from the network-perspective, it could be that damage to long-range connections more closely reflects the overall level of multiple sclerosis-related damage, and thus is a better marker for diffuse damage compared to more localized heterogeneous damage. In this study, especially conventional measures of structural damage were examined, future studies are needed to investigate more advanced measures of structural damage, such as the effect of changes in specific tissue compartments (i.e. normal-appearing white matter fractional anisotropy, lesional fractional anisotropy, the effect of the severity of a lesion) and the effect of lesion orientation relative to the direction of the tract on network efficiency and clinical measures.
Based on our cross-sectional data, we can, unfortunately, only speculate about the order of events. Until now, computational modelling studies have investigated the influence of structural changes and functional brain changes, and vice versa (Tewarie et al., 2014). Future longitudinal in vivo studies are needed to investigate the temporal evolution of the involvement of short- and long-range connections, their interplay, and the impact on structural and functional network efficiency.
Limitations
There are some methodological choices and issues to be considered. The results of tractography algorithms may be affected by the presence of white matter lesions. To assure the credibility of the tractography findings, we performed additional analyses. We found that the number of fibres, the length of the structural connections and the density of the structural matrices were not different between healthy controls and multiple sclerosis patients. In addition, in patients with multiple sclerosis, lesion load was not associated with the length of the observed structural connections (rho = 0.240; P = 0.784). Visual inspection showed that MRtrix was able to follow a white matter fibre through lesions in the white matter (Supplementary Fig. 1), as was also seen in other publications (Tur et al., 2018; Charalambous et al., 2019). Furthermore, a recent study investigating the impact of lesions on tractography methods reported that, although some impact of multiple sclerosis lesions is observed, tractography algorithms are still well able to anatomically localize tracts in multiple sclerosis (Lipp et al., 2019). Altogether, the above reasons support the assumption that it is unlikely that our findings are completely driven by differences in tractography methodology between healthy controls and patients with multiple sclerosis. In addition, it was recently suggested that the node definition by different parcellation schemes could produce different properties of brain networks (Hagmann et al., 2008; Zalesky et al., 2010). Future studies are therefore needed to investigate which tractography method and parcellation scheme is most closely related to a golden standard. In this study, we used the average healthy control matrix as reference to categorize the tracts in short- and long-range connections, especially because in this population the structural networks were not influenced by disease-related pathology. One could also argue the use of structural networks of patients with multiple sclerosis as a reference; however, the impact of this decision on our findings seems to be limited because of the very strong correlation between the tract lengths observed healthy controls and patients with multiple sclerosis (r = 0.995; P < 0.001). Another limitation could be the generalizability of our results. The study population consisted mostly of patients with long-standing multiple sclerosis, therefore, future studies should investigate whether there is a different relationship between damage to short- and long-range connections, network metrics and cognition in different phases of the disease. In addition, a future longitudinal study would be vital to demonstrate progressive alterations in structural network measures. Further, in this study we have chosen to investigate global efficiency as measure of interest to measure of the ability of a network to transmit information at the global level (Bassett and Bullmore, 2006); however, other graph measures, such as clustering coefficient and path length, could have provided additional insights and need to be investigated in future brain network studies. This study can be seen as a first step towards a better understanding of the structural brain architecture in patients with multiple sclerosis and bridging the gap between structural network properties, functional network alterations and the cognitive status of the multiple sclerosis patient.
Conclusion
Our results indicate that global integration of information, facilitated by long-range connections, is hampered in patients with long-standing multiple sclerosis. By contrast, local integration, mediated by short-range connections, is less affected. Shifting the network balance towards the short-range connections following predominant damage to long-range fibres was not only shown to reduce efficiency of the structural network, but also to impact cognitive function and the functional network. This novel, topology-based analysis of structural brain network pathophysiology provides a more integrative and neurobiologically plausible explanation for functional brain alterations and cognitive decline in multiple sclerosis.
Funding
This study was supported by the Dutch MS Research Foundation, grant numbers 08-650, 13-820 and 14-358e.
Competing interests
The authors report no competing interests.
Supplementary Material
References
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol 2007; 3: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CWM, Abdulla YH, Torres EM, Poston RN. Periventricular lesions in multiple sclerosis: their perivenous origin and relationship to granular ependymitis. Neuropathol Appl Neurobiol 1987; 13: 141–52. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist 2006; 12: 512–23. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET. Small-world brain networks revisited. Neuroscientist 2017; 35: 499–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercury KK, Macklin WB. Dynamics and mechanisms of CNS myelination. Dev Cell 2015; 32: 447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 2010; 14: 277–90. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci 2012; 13: 336–49. [DOI] [PubMed] [Google Scholar]
- Charalambous T, Tur C, Prados F, Kanber B, Chard DT, Ourselin S, et al. Structural network disruption markers explain disability in multiple sclerosis. J Neurol Neurosurg Psychiatry 2019; 90: 219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard DT, Jackson JS, Miller DH, Wheeler-Kingshott CAM. Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J Magn Reson Imaging 2010; 32: 223–8. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet 2008; 7: 1139–51. [DOI] [PubMed] [Google Scholar]
- Daams M, Steenwijk MD, Wattjes MP, Geurts JJG, Uitdehaag BMJ, Tewarie PK, et al. Unraveling the neuroimaging predictors for motor dysfunction in long-standing multiple sclerosis. Neurology 2015; 85: 248–55. [DOI] [PubMed] [Google Scholar]
- Dineen RA, Vilisaar J, Hlinka J, Bradshaw CM, Morgan PS, Constantinescu CS, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 2009; 132: 239–49. [DOI] [PubMed] [Google Scholar]
- Eijlers AJC, Meijer KA, van Geest Q, Geurts JJG, Schoonheim MM. Determinants of cognitive impairment in patients with multiple sclerosis with and without atrophy. Radiology 2018; 288: 544–51. [DOI] [PubMed] [Google Scholar]
- Eijlers AJC, Meijer KA, Wassenaar TM, Steenwijk MD, Uitdehaag BMJ, Barkhof F, et al. Increased default-mode network centrality in cognitively impaired multiple sclerosis patients. Neurology 2017; 88: 952–60. [DOI] [PubMed] [Google Scholar]
- Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 2000; 47: 391–5. [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci 2015; 16: 159–72. [DOI] [PubMed] [Google Scholar]
- Gallos LK, Makse HA, Sigman M. A small world of weak ties provides optimal global integration of self-similar modules in functional brain networks. Proc Natl Acad Sci 2012; 109: 2825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008; 6: e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider L, Zrzavy T, Hametner S, Hoftberger R, Bagnato F, Grabner G, et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 2016; 139: 807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SL, Bhan AK, Gilles F, Kemp M, Kerr C, Weiner HL. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol 1986; 19: 578–87. [DOI] [PubMed] [Google Scholar]
- He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain 2009; 132: 3366–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinski J, Stagg CJ, Chance SA, Deluca GC, Esiri MM, Chang EH, et al. A combined post-mortem magnetic resonance imaging and quantitative histological study of multiple sclerosis pathology. Brain 2012; 135: 2938–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005; 128: 2705–12. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol 2007; 17: 210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science 2003; 301: 1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp I, Parker G, Tallantyre E, Goodall A, Grama S, Patitucci E, et al. Tractography in the presence of white matter lesions in multiple sclerosis. bioRxiv 2019: 559708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer KA, Eijlers AJC, Douw L, Uitdehaag BM, Barkhof F, Geurts JJG, et al. Increased connectivity of hub networks and cognitive impairment in multiple sclerosis. Neurology 2017; 88: 2107–14. [DOI] [PubMed] [Google Scholar]
- Meijer KA, van Geest Q, Eijlers AJC, Geurts JJG, Schoonheim MM, Hulst HE. Is impaired information processing speed a matter of structural or functional damage in MS? NeuroImage Clin 2018; 20: 844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer KA, Muhlert N, Cercignani M, Sethi V, Ron MA, Thompson AJ, et al. White matter tract abnormalities are associated with cognitive dysfunction in secondary progressive multiple sclerosis. Mult Scler J 2016; 22: 1429–37. [DOI] [PubMed] [Google Scholar]
- Pardini M, Yaldizli Ö, Sethi V, Muhlert N, Liu Z, Samson RS, et al. Motor network efficiency and disability in multiple sclerosis. Neurology 2015; 85: 1115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H-J, Friston K. Structural and functional brain networks: from connections to cognition. Science 2013; 342: 1238411. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Stephan KE, Kötter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci 2002; 3: 606–16. [DOI] [PubMed] [Google Scholar]
- Perge JA, Niven JE, Mugnaini E, Balasubramanian V, Sterling P. Why do axons differ in caliber? J Neurosci 2012; 32: 626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010; 52: 1059–69. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 2014; 90:229–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nat Rev Neurol 2014; 10: 156–66. [DOI] [PubMed] [Google Scholar]
- Shu N, Liu Y, Li K, Duan Y, Wang J, Yu C, et al. Diffusion tensor tractography reveals disrupted topological efficiency in white matter structural networks in multiple sclerosis. Cereb Cortex 2011; 21: 2565–77. [DOI] [PubMed] [Google Scholar]
- Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics 2004; 2: 145–62. [DOI] [PubMed] [Google Scholar]
- Steenwijk MD, Daams M, Pouwels PJW, Balk LJ, Tewarie PK, Killestein J, et al. What explains gray matter atrophy in long-standing multiple sclerosis? Radiology 2014; 272: 832–42. [DOI] [PubMed] [Google Scholar]
- Steenwijk MD, Geurts JJG, Daams M, Tijms BM, Wink AM, Balk LJ, et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 2015: 139: 115–26. [DOI] [PubMed] [Google Scholar]
- Steenwijk MD, Pouwels PJW, Daams M, Van Dalen JW, Caan MWA, Richard E, et al. Accurate white matter lesion segmentation by k nearest neighbor classification with tissue type priors (kNN-TTPs). NeuroImage Clin 2013; 3: 462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK, Zamponi GW, van Minnen J, Geurts JJG. Will the real multiple sclerosis please stand up? Nat Rev Neurosci 2012; 13: 507–14. [DOI] [PubMed] [Google Scholar]
- Tewarie P, Steenwijk MD, Tijms BM,, Daams M, Balk LJ, Stam CJ, et al. Disruption of structural and functional networks in long-standing multiple sclerosis. Hum Brain Mapp 2014; 35: 5946–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Sporns O, Edelman GM. Measures of degeneracy and redundancy in biological networks. Proc Natl Acad Sci 1999; 96: 3257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 2007; 35: 1459–72. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol 2012; 22: 53–66. [Google Scholar]
- Tur C, Eshaghi A, Altmann DR, Jenkins TM, Prados F, Grussu F, et al. Structural cortical network reorganization associated with early conversion to multiple sclerosis. Sci Rep 2018; 8: 10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goni J, Sporns O. High-cost, high-capacity backbone for global brain communication. Proc Natl Acad Sci 2012; 109: 11372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci 2013; 17: 683–96. [DOI] [PubMed] [Google Scholar]
- Yu HJ, Christodoulou C, Bhise V, Greenblatt D, Patel Y, Serafin D, et al. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage 2012; 59: 3713–22. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Harding IH, Cocchi L, Yücel M, Pantelis C, et al. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage 2010; 50: 970–83. [DOI] [PubMed] [Google Scholar]
- Zeki S, Shipp S. The functional logic of cortical connections. Nature 1988; 335: 311–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data, not published in the article, will be shared on reasonable request from a qualified investigator.