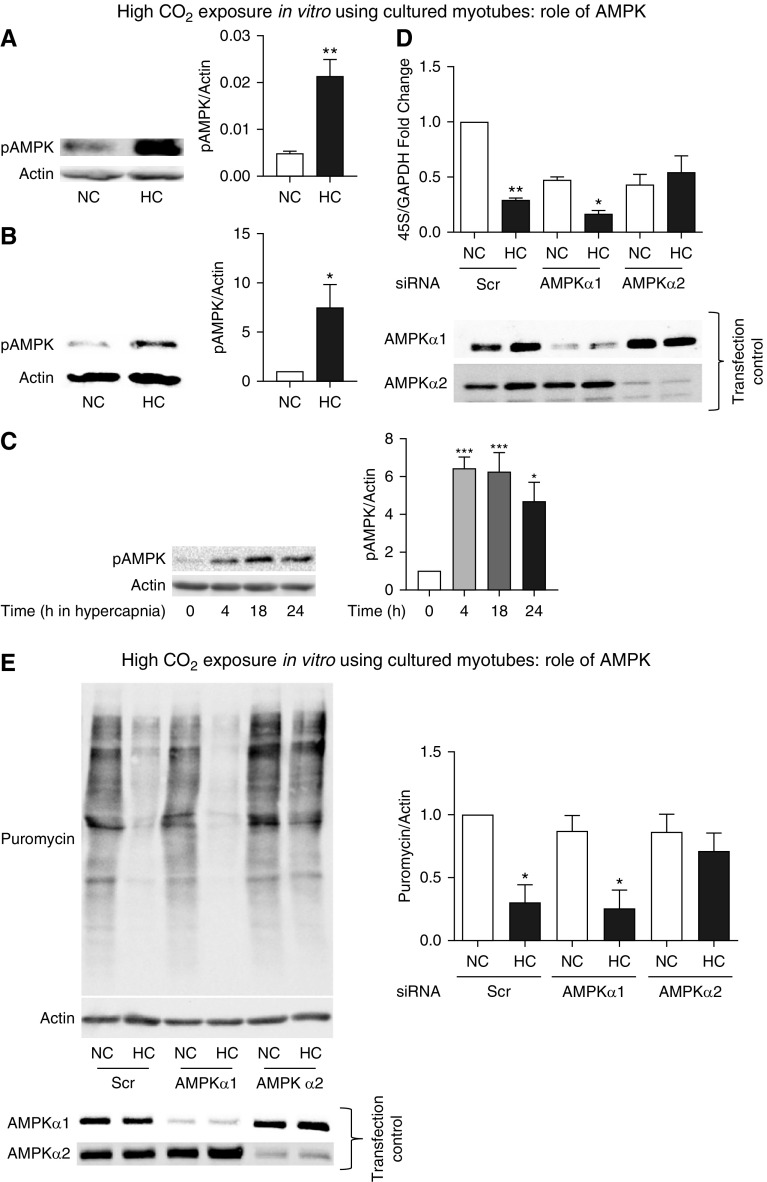
Figure 5.
AMP-activated protein kinase (AMPK) regulates protein anabolism during exposure to high CO2 in cultured myotubes. (A) AMPK phosphorylation (pAMPK) in skeletal muscle from mice exposed to high CO2 (HC) versus room air (NC) for 60 days. Actin was used as a lane loading control (n = 3). (B) pAMPK in primary myotubes exposed to 40 mm Hg (NC) or 120 mm Hg (HC) CO2 for 24 hours. Actin was used as a lane loading control (n = 3). (C) pAMPK in C2C12 myotubes exposed to 40 mm Hg (NC) or 120 mm Hg (HC) CO2 for different durations. Actin was used as a lane loading control. (D) Myotubes were transfected with scrambled (Scr), AMPKα1, or AMPKα2 siRNA and then exposed to normal (NC) or high CO2 (HC). Representative figures depict results of qPCR with primers to amplify 45S pre-RNA/GAPDH. Cell samples were processed for Western blotting and probed with specific antibodies to indicate AMPKα1 or AMPKα2 siRNA expression as a control of transfection and silencing efficiency in each condition (n = 3). (E) Conditions similar to D with myotubes exposed to NC and HC for 24 hours in the presence of puromycin-containing medium. Samples were then lysed and immunoblotted with antipuromycin antibody, and the intensity of the smears was scored as shown. Membrane was then stripped and probed with actin as a lane loading control. Transfection control was carried out by Western blotting and probing with specific antibodies to indicate specific silencing of AMPKα1 or AMPKα2 siRNA (n = 3). *P < 0.05, **P < 0.01, and ***P < 0.001.