Abstract
Arthritis, an inflammatory condition that causes pain and cartilage destruction in joints, affects over 54.4 million people in the US alone. Here, for the first time, we demonstrated the emerging role of Neural EGFL like 1 (NELL-1) in arthritis pathogenesis by showing that Nell-1- haploinsufficient (Nell-1+/6R) mice had accelerated and aggravated osteoarthritis progression with elevated inflammatory markers in both spontaneous primary osteoarthritis (OA) and chemical-induced secondary OA models. In the chemical-induced OA model, intra-articular injection of interleukin (IL)1β induced more severe inflammation and cartilage degradation in the knee joints of Nell-1+/6R mice than in wildtype animals. Mechanistically, in addition to its pro-chondrogenic potency, NELL-1 also effectively suppressed the expression of inflammatory cytokines and their downstream cartilage catabolic enzymes by upregulating runt-related transcription factor (RUNX)1 in mouse and human articular cartilage chondrocytes. Notably, NELL-1 significantly reduced IL1b-stimulated inflammation and damage to articular cartilage in vivo. In particular, NELL-1 administration markedly reduced the symptoms of antalgic gait observed in IL1β-challenged Nell-1+/6R mice. Therefore, NELL-1 is a promising pro-chondrogenic, anti-inflammatory dual-functional disease-modifying osteoarthritis drug (DMOAD) candidate for preventing and suppressing arthritis-related cartilage damage.
Keywords: Cartilage damage, Disease-modifying osteoarthritis drug (DMOAD), Inflammation, Neural EGFL like 1 (NELL-1), Osteoarthritis, Runt-related transcription factor 1 (RUNX1)
Introduction
Arthritis appears in over 100 identified diseases that can damage any joint in the body, causing inflammation that results in pain, stiffness, swelling, and decreased motion [1, 2]. As the leading cause of disability among adults, arthritis has been diagnosed in more than 10 million people in the United Kindom [1] and approximately 54.4 million people in the United States [2, 3]. In particular, osteoarthritis (OA) is the most common form of arthritis and affects around 18% of women and 10% of men over the age of 60 [4]. Unfortunately, the traditional use of analgesia [5] is insufficient for curative treatment since it does not reduce inflammation and cartilage damage [5–7]. Multiple adverse side-effects in the musculoskeletal, cardiovascular, and gastrointestinal systems [8–10] challenge the use of glucocorticoids as safe arthritis treatments, and nonsteroidal anti-inflammatory drugs (NSAIDs) reduce pain and inflammation in the short-term but do not effectively control arthritis progression [6]. Even more disappointing, the efficacy of disease-modifying antirheumatic drugs (DMARDs) that postpone rheumatoid arthritis (RA) progression by slowing or suppressing inflammation has not been replicated in OA clinical trials via systemic or local administration [11–13]. This likely occurs because these therapeutics do not directly manage cartilage destruction—the primary cause of OA [4, 6, 7, 14].
In order to combat the cartilage destruction seen in OA [4], the recent search for new OA therapeutics is shifting from synthetic chemicals to biological molecules, with a specific focus on pro-chondrogenic growth factors [7, 15–20]. For instance, neural EGFL-like 1 (NELL-1) is a novel pro-chondrogenic molecule that enhances the proliferation, chondrogenic differentiation, and maturation of chondrogenic-committed cells and their progenitors in vitro [21, 22]. As an extracellular matrix (ECM) molecule expressed in articular cartilage [21], NELL-1 alone is sufficient to promote cartilage regeneration without osteophyte formation in rabbit knee criticalsized, full-thickness subchondral defects [23]. Moreover, our recent studies identified a novel signaling cascade of NELL-1 → nuclear factor of activated T-cells (NFATc)1 → runt-related transcription factor (RUNX)3 → Indian hedgehog (IHH) in articular chondrocytes, which is essential for NELL-1’s pro-chondrogenic bioactivities [22, 24, 25]. Inspired by the genome-wide association study (GWAS) that associated single nucleotide polymorphisms (SNPs) within the NELL-1 gene with ankylosing spondylitis and psoriatic arthritis [26–28], the current study is intended to determine the role of NELL-1 in the pathogenesis of OA and its potential therapeutic benefits.
Materials and Methods
In silico prediction
In vitro half-life (t1/2) and the time required for reduction to 10% of the original mature human NELL-1 protein content (t90) in mammalian cells were predicted by the online server ProtParam (https://web.expasy.org/protparam/). The NELL-1 t1/2 is approximately 1.1 h and the t90 is less than 6 h in mammalian cells in vitro. These predictions were further confirmed by ProtLifePred (http://protein-n-end-rule.leadhoster.com/).
Genomatix Software v3.10 (Genomatix AG, Munich, Germany) was used to predict the potential binding motifs of RUNX1 and NFATc1 in chondrocytes/cartilage. The sites were computationally projected with predefined transcriptional factor binding modules [29].
Human arthritic cartilage
Human arthritic cartilage samples were obtained from patients of both sexes between the ages of 32 and 92 undergoing knee arthroplasty with an institutional review board (IRB) exemption since no donor identities were provided for these samples. These pre-fixed samples were used for histological and immunobiological analyses only. Meanwhile, primary adult human articular chondrocytes (hARCs) isolated from normal/healthy (NM), OA, and RA donors were purchased from Cell Application Inc. (San Diego, CA, US) and cultured according to the manufacturer’s instructions for in vitro investigations.
Animal maintenance
All the experiments on live mice were performed under an institutionally approved protocol provided by the Chancellor’s Animal Research Committee at UCLA (protocol numbers: 2014-041 and 2013-013). Due to N-ethyl-N-nitrosourea (ENU)-induced homozygous Nell-1-deficient (Nell-16R/6R) mice having a severely reduced expression of Nell-1 that results in neonatal death [30], Nell-1-haploinsufficient (Nell-1+/6R) mice (a well-established loss-of-function model [22, 24, 25]) were examined in the current investigation. Mice were bred and maintained as previously described [22, 24, 25], and their genotypes were determined by polymerase chain reaction (PCR). Genetic knockdown of Nell-1 was also confirmed by immunofluorescence (IF) staining in the tibia cartilage of newborn Nell-1+/6R mouse knees (Supplementary Fig. 1).
Primary osteoarthritis model
Slowly progressing OA in animals, such as in mice, closely simulates the natural progression of human primary OA [31]. Wild-type (WT) and Nell-1+/6R mice at 1 month old (a prepubescent stage for mice that developmentally approximates 12.5 years of age in humans [32, 33]), 3-months-old (a young mature adult stage for mice that developmentally approximates 20 years of age in humans [33]), and 18-months-old (a senescent stage for mice that developmentally approximates >50 years of age in humans [33]) were used to understand Nell-1’s activities in the pathogenesis of OA. Since the prevalence of OA in human is significantly higher in women than men [4], female mice were chosen for this proof-of-concept study. Special focus was directed to the medial tibial plateau area, which is one of the most critical loadbearing areas in the body [34]. The mice were euthanized with an overdose of phenobarbital (Piramal Healthcare, Maharashtra, India), and their right hind limbs were harvested for histological and IF staining. Expression levels of Nell-1 in the medial tibial plateau cartilage were monitored using laser-capture microdissection (LCM)-coupled quantitative real-time PCR (Supplementary Fig. 2) as described below.
Secondary osteoarthritis model
An imbalance in chondrocyte functions, which can be induced by outside stimuli such as the presence of inflammatory cytokines, leads to the progression of degenerative conditions like OA. In particular, the local elevation of IL1β in rodent knee joints has been shown to induce OA-like symptoms [35–37]. Thus, a well-documented modified direct mouse intra-articular IL1β injection model [38] was used to simulate secondary OA-like damage in vivo [31]. Briefly, under isoflurane anesthesia (5% for anesthesia induction and 2% for maintenance), a Hamilton syringe with a 29-gauge needle was inserted through the patellar ligament into the joint space of the right knee of 2.5-month-old female WT or Nell-1+/6R mice. Since the in vivo elimination t1/2 of NELL-1 is 5.5 h [39], the intra-articular injection was performed twice daily by the same surgeon to avoid variation in technique. For each genotype, animals were randomly assigned to the four treatment groups (6 mice per group; Fig. 1) before the first injection: ‘Control’ group: 6 μl phosphate-buffered saline (PBS) per injection for 14 days; ‘NELL-1’ group: 6 μl PBS per injection for 7 days followed by 2 μg recombinant human NELL-1 (Aragen Bioscience, Inc., Morgan Hill, CA, US) in 6 μl PBS per injection for another 7 days; ‘IL1β’ group: 100 ng recombinant human IL1β (PeproTech, Inc., Rocky Hill, NJ, US) in 6 μl PBS per injection for 14 days; and ‘IL1β + NELL-1’ group: 100 ng IL1β in 6 μl PBS per injection for 7 days to trigger the inflammation, while 100 ng IL1β + 2 μg NELL-1 in 6 μl PBS per injection was administered for an additional 7 days. It is worth noting that, in the ‘IL1β + NELL-1’ group, IL1β was injected along with NELL-1 in the second 7 day injection period to avoid the influence of spontaneous cartilage recovery after withholding IL1β, which was previously observed [35, 40, 41], and to more accurately mimic the pathological OA condition in which inflammatory stimulation is persistent. For gait analyses, videos were captured on day 7 and 14 and evaluated independently by three experienced physicians in a blinded fashion before the animals were sacrificed for histological analysis. An established gait scoring system, which was previously used in an inflammatory monoarthritic mouse model [42], was adapted to examine impacts on animal behavior (Supplementary Table 1) as recommended [43].
Fig. 1. Schematic depicting the intra-articular injection animal model.
‘Control’ group: 6 μl PBS per injection for 14 days; ‘NELL-1’ group: 6 μl PBS per injection for 7 days followed by 2 μg recombinant human NELL-1 in 6 μl PBS per injection for the next 7 days; ‘IL1β’ group: 100 ng recombinant human IL1β in 6 μl PBS per injection for 14 days; and ‘IL1β + NELL-1’ group: 100 ng IL1β in 6 μl PBS per injection for 7 days to trigger inflammation, and 100 ng IL1β + 2 μg NELL-1 in 6 μl PBS per injection for the next 7 days. All injections were performed twice daily.
Histological and IF staining
The mouse hind limb and human arthritic cartilage samples were fixed in 4% paraformaldehyde (MilliporeSigma; Burlington, MA, US) at 4°C for 24 h and decalcified with 19% ethylenediaminetetraacetic acid (pH 8.0; MilliporeSigma) for 21 days prior to paraffin embedding and sectioning at a thickness of 5 μm. Hematoxylin and eosin (H&E) staining was performed for histological analysis, while safranin O staining was conducted with the NovaUltra™ Safranin O staining Kit (IHC World, LLC, Woodstock, MD, US) according to the manufacturer’s instructions. Primary antibodies against type II collagen (N-N6B3, 1:20; Developmental Studies Hybridoma Bank, Iowa City, IA, US), IL1β (ab9722, 1:400; Abcam, Cambridge, MA, US), IL6 (TA500067, 1:50; Origene, Rockville, MD, US), matrix metallopeptidase (MMP)13 (ab39012, 1:200; Abcam), bone morphogenetic protein (BMP)6 (ab155963, 1:200; Abcam), BMP7 (ab84684, 1:1000; Abcam), RUNX1 (ab35962, 1:1000, Abcam), and NELL-1 (ABP-PAB-11648, 1:75; Allele Biotech, San Diego, CA, US) were used for IF staining. The Vector® M.O.M.™ Immunodetection Kit (Vector Laboratories, Inc., Burlingame, CA, US) was employed to locate mouse primary antibodies on mouse tissue. 4’,6-diamidino-2-phenylindole (DAPI; MilliporeSigma) was used for nuclear counterstaining.
Laser-capture microdissection (LCM)
For LCM, fresh-cut tissue sections at 10 mm were mounted on polyethylene naphthalate (PEN) Membrane Glass Slides (2.0 μm, MicroDissect GmbH, Herborn, Germany). To visualize the medial tibial plateau area, tissue sections were stained with Cresyl Fast Violet and completely air dried before microdissection [44]. Tibial cartilage was microdissected on a Leica LMD7000 system (Leica, Buffalo Grove, IL, US). After microdissection, the excised region was examined microscopically (Supplementary Fig. 3) and was kept on ice until RNA isolation.
In vitro mechanism of action investigation
To gain initial insight into the mechanism underlying NELL-1’s anti-arthritic bioactivities, primary articular cartilage chondrocytes were utilized for the in vitro investigation because: (1) chondrocytes are primary contributors to articular cartilage structural support, metabolic activities, and critical maintenance functions, such as ECM secretion, within joints [45]; (2) chondrocytes are a confirmed cell source that secretes NELL-1 [21, 22, 24, 25]; (3) local secretion of proinflammatory cytokines, including IL1β, IL6, and tumor necrosis factor (TNF)α [46–49] by articular chondrocytes can activate their autocrine loops, which are essential for the initiation and progression of arthritis [50–53]; and (4) the regulatory roles of these proinflammatory cytokines are at least partially independent from those of synovial and immune cells [53]. In particular, previous studies have confirmed that proinflammatory cytokines, including IL1β, can induce arthritis-like biological changes in articular chondrocytes in vitro [53]. Expression of proinflammatory cytokines IL1β, IL6, and TNFα, their downstream catabolic markers MMP13 and ADAM metallopeptidase with thrombospondin type 1 motif (ADAMTS)5, which are major contributors to ECM degradation during arthritis progression [54, 55], the chondrocyte-secreted inflammatory maker prostaglandin-endoperoxide synthase (PTGS)2 [53], and the anabolic marker type II collagen (encoded by COL2α1) were evaluated. Based on aforementioned in silico predictions, all assessments were conducted in the 6 h post-treatment window since the t90 of NELL-1 was estimated to be less than 6 h in mammalian cells.
Primary mouse articular chondrocyte isolation and cultivation
Primary mouse articular cartilage chondrocytes (mARCs) were isolated and cultivated as previously described [56–58]. Briefly, small pieces of articular cartilage, located at distant sites from the synovial tissue, were removed from 3-month-old female WT or Nell-1+/6R mice. These cartilage tissues were digested in 1.5 mg/ml collagenase B (MilliporeSigma) at 37°C overnight to achieve single-cell suspensions. After rinsing with Dulbecco’s Modified Eagle’s Medium (DMEM), mARCs were cultured in a basal culture medium [DMEM with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin]. The medium was changed every 3 days, and the cells were passaged at 70~90% confluence. All of these cell culture reagents were purchased from Thermo Fisher Scientific (Canoga Park, CA, US).
Gene expression analysis
Chondrogenic-committed ATDC5 cells were obtained from the RIKEN Cell Bank (Tsukuba, Japan) and cultured in DMEM/Ham’s F-12 medium (Thermo Fisher Scientific) containing 5% FBS. Passage 2 primary mARCs, isolated from 3-month-old female WT and Nell-1+/6R mice, and commercially available primary hARCs were also used for gene expression analysis. Subconfluent cells were subjected to serum starvation (0.1% FBS) for 18 h and stimulated with 10 ng/ml recombinant human IL1α with or without 0.8 or 2.0 μg/ml recombinant human NELL-1.
Total RNA isolated by the RNeasy® Mini Kit (for ATDC5 cell, mARCs, or hARCs; Qiagen, Germantown, MD, US) or the RNeasy® FFPE Kit (for mouse cartilage samples obtained by LCM; Qiagen) with DNase treatment was used for reverse transcription with the iScript™ Reverse Transcription Supermix for RT-qPCR (Bio-Rad Laboratories Inc., Hercules, CA, US). In particular, tibial cartilage samples collected from two animals were pooled together for RNA isolation to obtain enough RNA for analysis. One μl of reverse transcription product was used for real-time PCR with SsoAdvanced™ Universals Probes Supermix (Bio-Rad Laboratories Inc.) and TaqMan® primers/probe sets (Supplementary Table 2; Thermo Fisher Scientific) on a QuantStudio3 system (Thermo Fisher Scientific). For each individual real-time PCR assay, three independent reserve transcription reaction products were used as templates and tested in duplicate.
Enzyme-linked immunosorbent assay (ELISA)
The mouse IL-6 Platinum ELISA Kit (Cat. # BMS603–2), mouse TNF alpha Uncoated ELISA Kit (Cat. # 88–7324), human IL-6 ELISA Kit (Cat. # BMS213–2), human TNF alpha Uncoated ELISA Kit (Cat. # 88–7346), and human MMP-13 ELISA Kit (Cat. # EHMMP13) were purchased from Thermo Fisher Scientific, while the mouse Mmp13 ELISA Kit was purchased from MyBioSource.com (Cat. # MBS2884671; San Diego, CA, US). Five x 104 cell/well in 24-well plates were treated with 1 ml medium. Cell culture medium was collected 6 h post-treatment for ELISA analyses according to the manufacturers’ instructions.
Reduced representation bisulfite sequencing (RRBS)
RRBS was conducted by the Technology Center for Genomics & Bioinformatics at UCLA. Briefly, gDNA was extracted from NM-, OA-, and RA-hARCs using the AllPrep DNA/RNA/Protein Mini Kit (Qiagen). Library preparation began by using the Nextflex® Bisulfite-Seq Library Prep Kit followed by a MspI restriction enzyme digestion (PerkinElmer, Waltham, MA, US). First, digestion was performed, end-repair and ligation of Met-Seq adapters followed, and size selection occurred subsequently. Bisulfite conversion was performed using the EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA, US). The subsequent step consisted of PCR amplification for 17 cycles. This library was sequenced on a 150 bp, pair-end, HiSeq 3000 (Illumina, San Diego, CA, US) sequencing run. Data quality checks were performed on the Illumina SAV. Demultiplexing was performed with the Illumina Bcl2fastq2 v2.17 program. Sequencing data were aligned to the GRCh37 (hg19) genome via Bismark [59]. Alignment was quantified and translated to total CpG count using Bismark’s Methylation Extractor module. More than 90% of the reads were aligned to the genome (Supplementary Table 3), which was in the standard range for RRBS. Differential methylation was performed using diffmeth and annotation was performed with identgeneloc, which are modules that are included in the DMAP package [60].
RNA interference (RNAi)
Plasmid packages harboring shRNA that targeted mouse Runx1 (G151145_0-3) and Nfatc1 (TG5010315_A-D), respectively, were purchased from OriGene (Rockville, MD, USA) and used to create stable ATDC5 knockdown cell lines with 4 μg/ml puromycin (Thermo Fisher Scientific) following the ‘Application Guide’ provided by the vendor. A scramble control vector (TR30013) provided by OriGene was also used to establish a stable control ATDC5 cell line.
Statistics
All statistical analyses were conducted in consultation with the UCLA Statistical Biomathematical Consulting Clinic. The sample size for each individual experiment is presented in the respective figure legends. For the gene expression and ELISA assays, one-way ANOVA and two-sample t-test analyses were performed by OriginPro 8 (Origin Lab Corp., Northampton, MA, USA), while the Kruskal-Wallis ANOVA, Mann-Whitney U test, or paired-sample Wilcoxon test were used to analyze gait scoring. For all data presented in this manuscript, P < 0.05 (*) was considered a suggestive difference, while P < 0.005 (**) was recognized as a statistically significant difference based on a recent recommendation [61].
Results
Nell-1-haploinsufficiency is prone to arthritis-like pathologic changes with increased proinflammatory cytokines in mouse knee articular cartilage.
Similar to humans, mice naturally develop OA during maturation, which qualifies them as a primary OA model to study inflammation in joints [31]. At 1 month old, there were no apparent differences in cartilage degeneration or inflammation between the knees of WT and Nell-1+/6R mice (Supplementary Figs. 4 and 5) except that Nell-1+/6R tibial cartilage chondrocytes had slightly reduced Col2α1 expression accompanied by increased ll6 transcription (Supplementary Fig. 5B and D). Pathologically, the thicknesses of the entire articular cartilage were similar in both WT and Nell-1+/6R mice. However, unlike WT mice whose uncalcified hyaline cartilage (HC) above the tidemark constituted the major portion of the cartilage, Nell-1+/6R mice had cartilage in which the superficial HC and underlying calcified cartilage (CC) layer below the tidemark presented similar thicknesses, indicating that CC already expanded during the early adolescence period of Nell-1+/6R mice [32].
At 3 months of age, focal wear and tear of HC with early chondrocyte clustering were only observed in Nell-1+/6R mice, accompanied by a lower HC/CC ratio than in age-matched WT counterparts (Fig. 2A). In comparison with WT cartilage, Nell-1+/6R cartilage not only had significant type II collagen reduction and Mmp13 elevation at both the protein and RNA levels that represent key events in OA progression [54], but also had remarkedly pronounced Il1β and Il6 expression, which indicates elevated inflammation (Fig. 2A and Supplementary Fig. 5).
Fig. 2. Characterization of WT and Nell-1-haploinsufficient (Nell-1+/6R) mouse knee joints at 3 and 18 months of age.
Representative photos of 3- (A), and 18-month- (B) old female WT and Nell-1+/6R mouse knee joints. H&E staining was performed for histological analysis, while safranin O was used to stain proteoglycans. Expression of anabolic marker type II collagen (Collagen, type II), catabolic marker Mmp13, as well as proinflammatory markers interleukin (Il)1β and Il6 was evaluated by IF staining. DAPI was used for nuclear counterstaining. HC: uncalcified hyaline zone of articular cartilage; CC: calcified zone of articular cartilage. Solid arrows indicate the erosion in HC; open triangles indicate severe loss of HC. Bar = 500 μm. Relative RNA expression in the tibial cartilage is presented in Supplementary Fig. 5.
At 18 months of age, limited wear and tear was found in HC layer of WT cartilage with decreased type II collagen and increased Mmp13 and Il1β (Fig. 2B); a profile that exhibited great similarity to that of 3-month-old Nell-1+/6R mice. In contrast, severe loss of HC [containing almost completely absent proteoglycan, negligible type II collagen, but significantly upregulated Mmp13, Il1β, and Il6 (Fig. 2B and Supplementary Fig. 5)] and exposure of the underlying CC were observed in 18-month-old Nell-1+/6R mouse knees. These histological and immunological changes were similar to those seen in late-stage OA of human patients [62].
Taken together, encompassing the age spectrum from juvenile, young adult, to elderly, Nell1-haploinsufficiency drastically accelerated and aggravated the arthritis-like cartilage degeneration in mice and was accompanied with significant elevation of proinflammatory cytokines.
Intra-articular injection of IL1β induced exaggerated OA-like damage in Nell-1+/6R mouse knees.
As a secondary OA model [31], 7 days of intra-articular IL1β injection (Fig. 1) was sufficient to induce proteoglycan degradation and upregulate proinflammatory cytokines Il1β and Il6 in HC of articular cartilage of 2.5-month-old WT mice (Supplementary Fig. 6). Continuously challenging the joints with IL1β for 14 days led to more severe arthritis-like damage, as characterized by (1) complete abolishment of proteoglycan expression on the tibial and femoral cartilage, (2) minimal staining of type II collagen on both HC and CC, (3) increased expression of Mmp13 in HC, and a significant boost in Il1β and Il6 levels in HC (Fig. 3A: ‘IL1β’, and Supplementary Fig. 7A–D). However, this damage was not severe enough to significantly alter the mobility of WT mice (Supplementary Fig. 8A). In comparison with age-matched WT counterparts, IL1β injection for 7 days in 2.5-month-old Nell-1+/6R mouse knees resulted in a more advanced decrease of proteoglycan and type II collagen, as well as bursts of increased Mmp13, Il1β, and Il6 levels (Supplementary Fig. 6). More importantly, 14 days of IL1β injection in 2.5-month old Nell-1+/6R mice (3 months old at the end of treatment) replicated the drastic arthritis-like damage seen in 18-month old Nell-1+/6R mice with regard to HC erosion, proteoglycan degradation, and Mmp13 expression, in addition to even more severely reduced type II collagen and increased inflammation (Fig. 3B: ‘IL1β’, and Supplementary Fig. 7A–D). In congruence with the histological assessment, the symptoms of antalgic gait were observed among ‘IL1β’ group Nell-1+/6R mice (Supplementary Video 1: ‘IL1β’, and Supplementary Fig. 8B). Therefore, IL1β induced more severe arthritis-like damage in Nell-1+/6R mouse knees.
Fig. 3. Characterization of mouse knee joints after 14 days of intra-articular injections.
Representative photos of 2.5-month-old female WT (A) and Nell-1+/6R (B) mouse knee joints after 14 days of intra-articular injections (3 months old at the end of treatment). The injection schematic of each group is presented in Fig. 1. H&E staining was performed for histological analysis, while safranin O was used to stain proteoglycans. Expression of Collagen, type II, Mmp13, Il1β, and Il6 was evaluated by IF staining. DAPI was used for nuclear counterstaining. HC: uncalcified hyaline zone of articularx cartilage; CC: calcified zone of articular cartilage. Relative RNA expression in the tibial cartilage is presented in Supplementary Fig. 7, while gait scores are summarized in Supplementary Fig. 8. Corresponding videos of Nell-1+/6R mice (B) are provided in Supplementary Videos 1–7. Bar = 500 μm.
NELL-1 injection rescued IL1β-induced arthritis-like damage in adult mouse knees.
To estimate the potential therapeutic benefits of NELL-1 against arthritic damage, NELL-1 was administered in the aforementioned intra-articular injection model with or without an accompanying IL1β challenge (Fig. 1). In WT mice, in comparison with a PBS vehicle control (Fig. 3A: ‘Control’), NELL-1 injections alone slightly increased the amount of proteoglycan and type II collagen with less Il1β staining in HC (Fig. 3A: ‘NELL-1’). In Nell-1+/6R mice, NELL-1 alone upregulated proteoglycan and type II collagen deposition in knee cartilage, while simultaneously reducing the expression of Il1β and Il6 to comparable levels of those in the age-matched WT animals (Fig. 3B: ‘NELL-1’, and Supplementary Fig. 7).
When NELL-1 was administered with IL1β after the initial 7 days of IL1β-challenging, the IL1β-induced arthritis-like damage was partially rescued in WT animals, as detected by the presence of safranin O staining, upregulation of type II collagen density, and observation of IL1β and Il6 signals similar to those of the ‘Control’ group (Fig. 3A: ‘IL1β + NELL-1’, and Supplementary Fig. 7). Similarly, administration of exogenous NELL-1 significantly reduced the inflammatory response and damage to articular cartilage in IL1β-challenged Nell-1+/6R mice (Fig. 3B: ‘IL1β + NELL-1’. and Supplementary Fig. 7). Importantly, the symptoms of antalgic gait in ‘IL1β + NELL-1’ treated Nell-1+/6R mice were moderate, and far less severe than the symptoms observed in ‘IL1β’ group Nell-1+/6R animals (Supplementary Video 1: ‘IL1β + NELL-1’, and Supplementary Fig. 8B). Excitingly, in a second validation experiment that tracked the mobility of ‘IL1β + NELL-1’ group Nell-1+/6R animals, the antalgic gait driven by the 7-day IL1β-challenge was drastically reduced by the subsequent 7 days of exogenous NELL-1 and IL1β administration in all 6 tested Nell-1+/6R mice (Supplementary Videos 2–7 and Supplementary Fig. 8C). Collectively, our present data demonstrate that NELL-1 has possible therapeutic potential for preventing and controlling the pathogenesis of arthritis.
NELL-1 significantly reduced IL1β-stimulated expression of inflammatory and catabolic molecules in mouse and human articular chondrocytes in vitro.
In alignment with the aforementioned mouse models in which low Nell-1 levels correlated with high inflammation and vice versa, we observed that intense IL1β staining was generally accompanied by low levels of NELL-1, but was not necessarily associated with less BMP6 or BMP7, in human arthritic articular cartilage lesions (Supplementary Fig. 9). This observation encouraged us to hypothesize that, in addition to its pro-chondrogenic functions, NELL-1 may also directly reduce inflammation in both mouse and human arthritis.
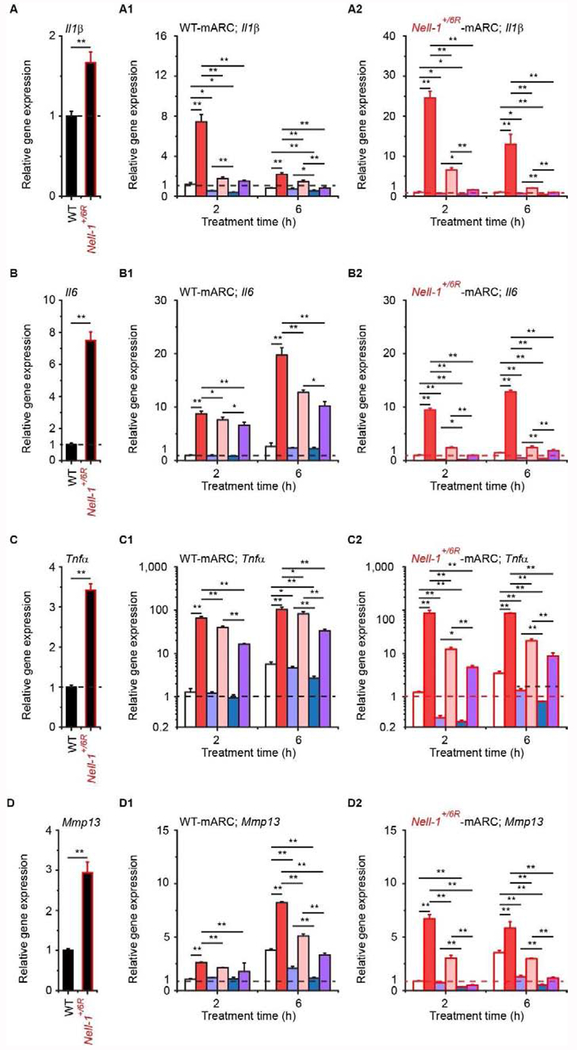
As expected, Nell-1-haploinsufficiency led to increased expression of proinflammatory and catabolic genes in primary mARCs (Fig. 4A–F). On the other hand, an apparent NELL-1 dose-dependent decline in proinflammatory and catabolic gene expression was generally observed when the mARCs were treated with NELL-1 protein alone (Fig. 4A1–F2). Meanwhile, IL1β significantly induced the transcription of all tested proinflammatory and downstream catabolic markers in both WT- and Nell-1+/6R-mARCs, which were consistently blocked by NELL-1 (Fig. 4A1–F2). The anti-inflammatory effects of NELL-1 on mARCs were further confirmed at the protein levels using ELISA (Supplementary Fig. 10). In comparison with WT-mARCs, Nell-1+/6R-mARCs also had lower levels of Col2α1 (which encodes the anabolic maker type II collagen; Fig. 4G), which further supports the pro-chondrogenic role of NELL-1. IL1β also markedly reduced Col2α1 expression in both WT- and Nell-1+/6R-mARCs, and its effects were seemingly more pronounced in Nell-1+/6R-mARCs (Fig. 4G1–G2), while a higher dose (2 μg/ml) of NELL-1 completely eliminated the downregulation of Col2α1 caused by IL1β-stimulation or Nell-1-haploinsufficiency (Fig. 4G1–G2). Interestingly, we also noticed that NELL-1 administration upregulated endogenous Nell-1 expression in mARCs (Fig. 4H–H2), which is in agreement with the results from the in vivo studies (Supplemental Fig. 7E). Taken together, these data reveal that NELL-1 could, at least partially, rescue mARCs from invoking chondrogenic ECM degradation and inflammation induced by endogenous Nell-1-deficiency or exogenous IL1β-stimulation.
Fig. 4. Effects of NELL-1 on gene expression in primary mouse articular chondrocytes (mARCs).
Expression of Il1β (A-A2), Il6 (B-B2), Tnfβ (C-C2), Mmp13 (D-D2), Adamts5 (E-E2), Ptgs2 (F-F2), Col2α1 (G-G2), and Nell-1 (H-H2) was quantified by real-time PCR, and the data were normalized to the respective levels of WT-mARCs (A-H and A1-H1) or Nell-1+/6R-mARCs (A2-H2) before treatment (dashed lines). Mean + SD of three independent experiments performed in duplicate are shown. One-way ANOVA and two-sample t-test analyses were performed. *, P < 0.05, a suggestive difference; **, P < 0.005, a statistically significant difference.
Furthermore, the aforementioned bioactivities of NELL-1 were also validated in primary hARCs isolated from NM, OA, and RA donors. In comparison with NM-hARCs, OA- and RA-hARCs had elevated levels of proinflammatory and catabolic markers, which were generally downregulated by exogenous NELL-1 application (Fig. 5A–F3). Due to the well-documented species-specific activity among mammalian IL1β [63, 64], hARCs, in comparison with mARCs, demonstrated a greater increase in proinflammatory and catabolic gene expression in response to recombinant human IL1β administration. Further, as expected, NELL-1 actively reduced the IL1μ-stimulated inflammatory responses in hARCs (Fig. 5A1–F3 and Supplementary Fig. 11). Collectively, our current data indicate that, as a pro-chondrogenic agent, NELL-1 also has a protective function against inflammation in chondrocytes, and thus, has the potential to be used as a disease-modifying osteoarthritis drug (DMOAD).
Fig. 5. Effects of NELL-1 on gene expression in primary human articular chondrocytes (hARCs).
Expression of IL1β (A-A3), IL6 (B-B3), TNFα (C-C3), MMP13 (D-D3), ADAMTS5 (E-E3), PTGS2 (F-F3), COL2α1 (G-G3), and NELL-1 (H-H3) was quantified by real-time PCR, and the data were normalized to the respective levels of pathological normal/health (NM)-hARCs (A-H and A1-H1), osteoarthritis (OA)-hARCs (A2-H2), or rheumatoid arthritis (RA)-hARCs (A3-H3) before treatment (dashed lines). Mean + SD of three independent experiments performed in duplicate are shown. One-way ANOVA and two-sample t-test analyses were performed. *, P < 0.05, a suggestive difference; **, P < 0.005, a statistically significant difference.
Additionally, less COL2α1 was expressed by OA- and RA-hARCs in comparison with NM-hARCs; COL2α1 transcription was reduced by IL1β and increased by NELL-1 in hARCs, which was similar to the effects of IL1β and NELL-1 seen in mARCs (Fig. 5G–G3). Meanwhile, OA- and RA-hARCs have significantly lower levels of NELL-1 than NM-hARCs (Fig. 5H), while RA-hARCs also exhibited higher CpG methylation levels at the NELL-1 locus than NM-hARCs (Supplementary Table 4), suggesting NELL-1 may also be involved in the pathogenesis of RA.
RUNX1 mediates NELL-1’s anti-inflammatory activities.
Previous studies demonstrate that NFATc1 and RUNX1 are primary genes that respond to NELL-1 in chondrocytes [25]. An in silico bioinformatic prediction indicates that both NFATc1 and RUNX1 potentially bind to promoters of IL1β and/or TNFα in human and mouse cartilage/chondrocytes (Supplementary Table 5). Since the anti-inflammatory effects of NELL-1 on primary mARCs and hARCs were replicated in ATDC5 cells (Supplementary Fig. 12), RNAi was used to establish stable Nfatc1- and Runx1-knockdown (KD) ATDC5 cells, respectively (Supplementary Fig. 13), to examine whether RUNX1 and/or NFATc1 mediate NELL-1’s anti-inflammatory activities in chondrocytes. In agreement with previous observations that both RUNX1 and NFATc1 are negative regulators of inflammation in arthritic conditions [65–68], Nfatc1- and Runx1-KD ATDC5 cells had higher endogenous levels of Il1β, Il6, and Tnfα (Fig. 6A–C). Moreover, IL1β administration induced more pronounced elevation of these proinflammatory genes in Nfatc1- and Runx1-KD ATDC5 cells than in the scramble control plasmid transfected (scramble) ATDC5 cells (Fig. 6A1–C3).
Fig. 6. Effects of Nfatc1- and Runx1-KD on NELL-1’s anti-inflammatory potency.
Stable scramble, Nfatc1-, and Runx1-KD ATDC5 clones were established (Supplementary Fig. 13). Expression of proinflammatory genes Il1β (A-A3), Il6 (B-B3), and Tnfα (C-C3) was quantified by real-time PCR, and the data were normalized to the respective levels of ATDC5 (A-C), ATDC5 (scramble) (A1-C1), ATDC5 (Nfatc1-KD) (A2-C2), or ATDC5 (Runx1-KD) (A3-C3) cells before treatment (dashed lines). Mean + SD of three independent experiments performed in duplicate are shown. One-way ANOVA and two-sample t-test analyses were performed. N.D.: not detectable. *, P < 0.05, a suggestive difference; **, P < 0.005, a statistically significant difference.
NELL-1’s anti-inflammatory effects were conserved in the Nfatc1-KD ATDC5 cells (Fig. 6A2–C2) at the same level as in the scramble ATDC5 cells (Fig. 6A1–C1). On the contrary, Runx1-KD almost completely eliminated NELL-1’s anti-inflammatory bioactivity in ATDC5 cells (Fig. 6A3–C3 and Supplementary Fig. 14). Interestingly, in comparison with non-transfected or scramble ATDC5 cells, although Runx1 upregulation was largely reduced and postponed in Runx1-KD ATDC5 cells (Supplementary Fig. 15), the leaking Runx1 elevation induced by a high dose (2 μg/ml) of NELL-1 at 6 h post-treatment was still sufficient to markedly weaken the IL1β-responsive expression of Il1β in Runx1-KD ATDC5 cells (Fig. 6A3). This phenomenon further supports the hypothesis that RUNX1 is essential and adequate to render NELL-1’s anti-inflammatory activity in chondrocytes. We also observed that Nell-1+/6R mARCs had decreased Runx1 expression (Supplementary Fig. 16A), while OA- and RA-hARCs had lower RUNX1 levels in comparison with NM-hARCs (Supplementary Fig. 17A). Moreover, NELL-1 significantly upregulated Runx1/RUNX1 in all tested primary mARCs and hARCs in vitro (Supplementary Fig. 16B–C and Supplementary Fig. 17B–D). This gene expression alteration has been further confirmed at the protein level in the aforementioned intra-articular injection model in vivo: intra-articular NELL-1 administration upregulated Runx1 protein in mouse knee cartilage, which was not altered by the absence or presence of IL1β-stimulation or by Nell-1-haploinsufficiency alone (Fig. 7 and Supplementary Fig. 7F). Taken together, these data suggest that RUNX1, instead of NFATc1, is a key downstream mediator of NELL-1’s anti-inflammatory bioactivity in chondrocytes (Fig. 8).
Fig. 7. Expression of Runx1 in mouse knees with intra-articular NELL-1 administration.
Using IF, Runx1 expression was observed in 2.5-month-old female WT (A) and Nell-1+/6R (B) mouse knee joints after 14 days of intra-articular injections (3 months old at the end of treatment). DAPI was used for nuclear counterstaining. HC: uncalcified hyaline zone of articular cartilage; CC: calcified zone of articular cartilage. Bar = 500 μm.
Fig. 8. Schematic depicting NELL-1’s effects in articular cartilage.
(A) Focal wear and tear of HC with early chondrocyte clustering became evident in the tibial plateau cartilage of 3-month-old Nell-1+/6R mice, while severe loss of HC was observed in the knees of 18-month-old Nell-1+/6R mice. (B) Our previous studies revealed that the NELL-1 → NFATc1 → RUNX3 → IHH cascade in chondrocytes is responsible for NELL-1’s pro-chondrogenic bioactivities. Here, we demonstrate that RUNX1, instead of NFATc1, is essential for NELL-1 to exhibit its anti-inflammatory properties in chondrocytes.
Discussion
An ideal OA-combating agent that has the ability to safely reduce inflammation and promote cartilage regeneration has long been desired. The traditional use of analgesia is insufficient for curative treatment since it does not reduce inflammation and cartilage damage [5–7]; multiple adverse side-effects in the musculoskeletal, cardiovascular, and gastrointestinal systems [8, 9] challenge the use of glucocorticoids as safe arthritis treatments, and NSAIDs do not effectively control arthritis progression [6]. Even more disappointing, the efficacy of DMARDs that postpone RA progression by slowing or suppressing inflammation has not been replicated in OA clinical trials via systemic or local administration [11–13]. This likely occurs because these therapeutics do not directly manage cartilage destruction – the primary cause of OA [4, 5]. At this time, the prospect of using well-known pro-chondrogenic growth factors as treatments for arthritis does not appear to be optimistic either. For instance, administration of BMP7 can downregulate multiple cartilage catabolic molecules in OA-damaged tissue, but it does not notably alter proinflammatory cytokine expression [18]. Intra-articular injection of transforming growth factor (TGF)β even appears to further elevate inflammatory infiltration in treated joints [15, 16], while BMP6 can induce the production of proinflammatory cytokines, such as TNFα [19], from macrophages – a major cell type responsible for inflammation and destruction in OA-ridden synovium [17].
In addition to our recent studies that revealed and confirmed the important regulatory roles of NELL-1 in chondrogenic development and maturation [21–25], we also noticed a negative correlation between proinflammatory markers and NELL-1 in both mouse and human arthritic articular cartilage. Specifically, by using spontaneous primary OA and chemical-induced secondary OA models, we further demonstrated that Nell-1 -deficiency could accelerate and aggravate the progression of OA. Meanwhile, we documented a correlative decrease in NELL-1 expression with higher levels of proinflammatory cytokines found in OA-hARCs than NM-hARCs. To the best of our knowledge, this is the first time the emerging role of NELL-1 in arthritis pathogenesis has been elucidated.
Furthermore, RA-hARCs exhibited higher CpG methylation at the NELL-1 locus and lower NELL-1 transcription than NM-hARCs, which is in accordance with a previous microarray investigation that detected reduced NELL-1 expression in the damaged knee cartilage of anteromedial gonarthrosis patients [69]. In addition, SNPs within the NELL-1 gene have been detected in patients diagnosed with ankylosing spondylitis and psoriatic arthritis [26–28]. These phenomena indicate that NELL-1 may also be closely connected to the progression of a broad range of arthritis conditions; however, this observation should be further verified with a large number of arthritis patients. Moreover, determining whether alterations of NELL-1’s genetic, epigenomic, and transcriptional levels are consequences or causes of continuous inflammatory infiltration should be carefully delineated in the future.
With NELL-1-stimulated chondrogenic regeneration observed in articular cartilage defects in vivo, there is an expected benefit of NELL-1 when used as arthritis therapeutic [23]. For example, when healthy, CC remains relatively constant in articular cartilage since the chondrocytes within CC typically stay quiescent during adulthood [70]. In this study, we found that intra-articular NELL-1 injection led to a moderately thickened CC layer of the tibial plateau cartilage in both WT and Nell-1+/6R mice. Importantly, unlike CC reactivation in OA as a result of progressive calcification of the unmineralized cartilage that reduces the thickness of HC and the entire articular cartilage [70], NELL-1-induced CC expansion was not accompanied by noticeable HC reduction. Since recent studies revealed that articular cartilage contains mesenchymal stem cells (MSCs) and/or chondroprogenitors that are most abundant in, but not limited to, the superficial zone [71], NELL-1-induced CC expansion may result from its ability to stimulate the proliferation and chondrogenic differentiation of MSCs and chondroprogenitors [21], representing a wave of chondrogenesis in adult animals. In addition to its observed pro-chondrogenic effects, NELL-1 is able to downregulate the expression of proinflammatory and catabolic molecules, and as a result, demonstrate an anti-inflammatory potency in vitro and in vivo. Importantly, our current data demonstrate the potential of NELL-1 to rescue severe cartilage damage and reduce the symptoms of antalgic gait in an IL1β-challenged animal model that simulates OA pathogenesis, which could be attributed to NELL-1’s pro-chondrogenic and anti-inflammatory dual-potency (Fig. 8). Encouragingly, previous studies have not revealed any noticeable adverse effects when NELL-1 was investigated and used for treating osteoporosis, even with systemic delivery and chemical modification that dramatically prolongs its elimination t1/2 and distribution in the musculoskeletal system [39, 72]. Therefore, from both efficacy and safety standpoints, NELL-1 shows potential as a novel and promising DMOAD candidate in response to the unmet demand for OA therapeutics [20].
Until now, NFATc1 and NFATc2 are the most studied NELL-1 downstream effectors for chondrogenesis. Both NFATc1 and NFATc2 have been found to play important roles in maintaining cartilage health and repressing spontaneous OA [67, 68]. In particular, Nfatc2−/− mice develop OA between 12 to 24 months of age [73, 74], which is even more markedly accelerated by cartilage-specific ablation of Nfatc1 [67]. Despite this, the current understanding of the NFATc proteins’ actions in arthritis is unclear due to the controversial data. For instance, inhibition of calcineurin, an NFATc activator, decreased the severity of OA [75], while blocking glycogen synthase kinase 3β, an NFATc inhibitor, induced OA in mice [76]. Further, IL1β was found to induce the expression of NFATc1 in hARCs [77]. These observations differ from previously reported anti-arthritic effects of NFATc molecules [67, 68, 74]. By demonstrating that NFATc1 plays an essential role in mediating NELL-1’s pro-chondrogenic bioactivities via activation of the IHH signaling pathway [22, 24, 25], but that it is not a prerequisite for NELL-1’s anti-inflammatory potency (Fig. 8), our studies provide unique insight into determining the intricate roles of NFATc1 in arthritis, which may reconcile these seemly conflicting observations.
RUNX1 is another arthritis susceptibility gene [78–80] that has been targeted for DMOAD development [65, 81, 82]. Aini et al. demonstrated that intra-articular injection of RUNX1 mRNA resulted in upregulated anabolic gene expression accompanied by lower Illβ levels in OA mouse articular cartilage [66]. Following our previous studies that identified RUNX1 as a NELL-1-responsive gene in chondrocytes [25], this study demonstrates that RUNX1 is a key negative inflammatory regulator mobilized by NELL-1 and plays an anti-inflammatory protective role in the development of OA (Fig. 8). To our knowledge, this is the first time in which a functional upstream activator of RUNX1 has been identified for its therapeutic potency in chondrocytes.
Nevertheless, the understanding of the NELL-1 → RUNX1 –I IL1β functional axis is incomplete. First, the signal transduction from NELL-1 to RUNX1 is largely unknown, which may be partly due to the limited knowledge of NELL-1’s specific cell surface receptor(s), associated protein(s), and downstream activators. NELL-1 may provide its function through different cell surface receptor(s) or co-receptor(s) in a cell-type- and develop-stage-dependent manner [83]. Meanwhile, the detailed mechanism of RUNX1 in arthritis is not yet clear [84, 85]. Moreover, we noticed that, although IL1β did not necessarily alter articular cartilage chondrocyte NELL-1 expression in the short timeframe after exposure, the presence of IL1β profoundly blocked endogenous NELL-1 upregulation that was expected in response to exogenous NELL-1 stimulation (Figs. 4H1–H2 and 5H1–H3). Given these facts, the interactions among NELL-1, RUNX1, and IL1β are far more complicated than they initially appear. Additionally, the mechanism behind the critical anti-arthritic autoinduction-like effect of NELL-1 in vitro (Figs. 4H1–H2 and 5H1–5H3) and in vivo (Supplementary Fig. 7E) is an interesting topic for subsequent investigation. Furthermore, the effects of NELL-1 on synovial and immune cells in the vicinity should also be assessed to fully elucidate the benefits of NELL-1 in arthritis management.
There appears to be no animal model used as the gold standard for osteoarthritis [86]. In the current study, a naturally occurring OA model gave the best representation of human primary OA and a chemical-induced model simulated human secondary OA to demonstrate the importance and potential therapeutical application of NELL-1 as a DMOAD. Chemical-induced OA models (such as the intra-articular IL1β injection model used in this study) are preferred for elucidating the genetic and molecular pathogenesis and identifying targets for drug therapy since they have no correlation to post-traumatic OA [31]. However, from a clinical aspect, the anti-arthritic efficacy of NELL-1 should be further confirmed in a post-traumatic OA model since both the tested models in this study do not simulate post-traumatic OA, which constitutes 12% of all symptomatic OA cases [87]. For instance, the surgical anterior (cranial) cruciate ligament transection (ACLT) model is the earliest and the most commonly used surgical model for simulating post-traumatic OA [31, 86]. Meanwhile, in comparison with small animals such as mice and rats, large animals have more anatomical and biomechanical similarities to humans [31,86]. In particular, goat knees have the closest anatomical resemblance to human knees [88]. Thus, a goat ACLT model may be useful for validating NELL-1’s anti-arthritic potency and provide more clinically relevant data. The occurrence of OA is significantly higher in women [4, 31], which prompted the use of female animals in our current proof-in-concept study. However, the effect of gender and reproductive status should also be evaluated in future translational studies. Lastly, the repeat intra-articular injection strategy that was used in this study is clearly not the optimal administration route for clinical treatment. Further optimization with regard to the dose and treatment regimen of NELL-1 administration should be conducted before NELL-1 can be used in clinical applications. Previous studies revealed that the in vivo elimination t1/2 of NELL-1 is only 5.5 h [39]; therefore, developing a suitable delivery vehicle and/or chemical modifications (such as PEGylation [39]) may also be needed to protect NELL-1 from endogenous enzyme digestion and subsequently elongate its biopotency in vivo. Taken together, considering that the investigation of NELL-1 in arthritis is in its infancy, a broad-range collaboration among academic, clinical, and therapeutic researchers is essential for facilitating the bench-to-bedside translation of this potential treatment.
Conclusions
In summary, by using a loss-of-function Nell-1+/6R mouse model, we demonstrated that NELL-1 has an anti-inflammatory role to protect articular cartilage from aggravated OA progression in addition to its previously exhibited pro-chondrogenic effects. Moreover, intra-articular injection of IL1β induced more severe inflammation and cartilage degradation in the knee joints of Nell-1+/6R mice than in WT control animals, while administration of exogenous NELL-1, used as a gain-of-function model, significantly reduced the inflammatory response and articular cartilage damage in both WT and Nell-1+/6R mouse knees. Excitingly, the heavy antalgic gait observed in IL1β-challenged Nell-1+/6R mice notably recovered after NELL-1 administration. The anti-inflammatory effects of NELL-1 were also replicated in vitro, as evidenced by strong repression of IL1β–stimulated inflammatory markers and their downstream catabolic enzymes that are responsible for cartilage ECM degradation. By taking advantage of RNAi technology, we demonstrate that RUNX1, instead of NFATc1, mediates the anti-inflammatory activities of NELL-1 in chondrocytes. Collectively, for the first time, our current study not only demonstrates the emerging role of NELL-1 in arthritis pathogenesis but also introduces NELL-1 as a promising new-generation DMOAD for preventing and suppressing arthritis-related cartilage damage on account of its pro-chondrogenic and anti-inflammatory potency, both of which are absent in currently available OA medications. Future investigation is strongly encouraged to uncover the detailed underlying mechanism and optimize the dose, regimen, and delivery method for transferring NELL-1-based therapies into clinical practice.
Supplementary Material
Acknowledgments
We would like to thank Mr. Jong Kil Kim for assisting with animal maintenance. RRBS analysis was conducted with the support of the Technology Center for Genomics and Bioinformatics at UCLA.
Funding
This study was financially supported by NIH-NIAMS Grants R01AR066782 and R01AR068835, and NIH-NCATS UCLA CTSI Grant (UL1TR001881). Laser-capture microdissection (LCM) was performed at the Advanced Light Microscopy/Spectroscopy Laboratory and the Leica Microsystems Center of Excellence at the California NanoSystems Institute at UCLA with funding support from NIH Shared Instrumentation Grant S10OD025017 and NSF Major Research Instrumentation grant CHE-0722519. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its supplementary information.
Competing interests
CL, ZZ, XZ, KT, and CS are inventors of NELL-1 related patents. XZ, KT, and CS are also founders and/or past board members of Bone Biologics Inc./Bone Biologics Corp., who sublicense NELL-1 patents from the UC Regents, who also hold equity in the company. CTC is an inventor of NELL-1 related patents filed from Oak Ridge National Laboratory (ORNL) and a founder of NellOne Therapeutics, Inc., which licensed NELL-1 related patent applications from ORNL. Bone Biologics Inc./Bone Biologics Corp. and NellOne Therapeutics, Inc. did not provide financial support for the current study. All of the other authors declare no conflict of interest.
References
- [1].Arthritis. NHS of UK; Available from: https://www.nhs.uk/conditions/arthritis/. [Google Scholar]
- [2].Arthritis types. CDC of US; Available from: https://www.cdc.gov/arthritis/basics/types.html. [Google Scholar]
- [3].Understanding arthritis. Arthritis Foundation; Available from: https://www.arthritis.org/about-arthritis/understanding-arthritis/. [Google Scholar]
- [4].International ORS. Osteoarthritis: A serious disease. Osteoarthritis Research Society International; 2016. p. 1–103. [Google Scholar]
- [5].Appleton CT. Osteoarthritis year in review 2017: Biology. Osteoarthritis And Cartilage. 2018;26:296–303. [DOI] [PubMed] [Google Scholar]
- [6].Scott DL. Arthritis in the elderly In: Fillit HM, Rockwood K, Woodhouse K, editors. Brocklehurst’s textbook of geriatric medicine and gerontology. 7th ed. Philadelphia: SAUNDERS, ELSEVIER; 2010. p. 566–576. [Google Scholar]
- [7].Li C, Zou M, Zheng Z. Current medication for osteoarthritis. Acta Scientific Orthopaedics. 2018;1:09–12. [Google Scholar]
- [8].Cooper C, Bardin T, Brandi ML, Cacoub P, Caminis J, Civitelli R, et al. Balancing benefits and risks of glucocorticoids in rheumatic diseases and other inflammatory joint disorders: New insights from emerging data. An expert consensus paper from the European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO). Aging Clinical And Experimental Research. 2016;28:1–16. [DOI] [PubMed] [Google Scholar]
- [9].Compston J Glucocorticoid-induced osteoporosis: An update. Endocrine. 2018;61:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Habib GS, Saliba W, Nashashibi M. Local effects of intra-articular corticosteroids. Clinical Rheumatology. 2010;29:347–356. [DOI] [PubMed] [Google Scholar]
- [11].Verbruggen G, Wittoek R, Cruyssen BV, Elewaut D. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: A double blind, randomised trial on structure modification. Annals of the Rheumatic Diseases. 2012;71:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chevalier X, Ravaud P, Maheu E, Baron G, Rialland A, Vergnaud P, et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and nsaids: A randomised, multicentre, double-blind, placebo-controlled trial. Annals of the Rheumatic Diseases. 2015;74:1697–1705. [DOI] [PubMed] [Google Scholar]
- [13].Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis & Rheumatism-Arthritis Care & Research. 2009;61:344–352. [DOI] [PubMed] [Google Scholar]
- [14].van Laar M, Pergolizzi JV Jr., Mellinghoff HU, Merchante IM, Nalamachu S, O’Brien J, et al. Pain treatment in arthritis-related pain: Beyond nsaids. The Open Rheumatology Journal. 2012;6:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Allen JB, Manthey CL, Hand AR, Ohura K, Ellingsworth L, Wahl SM. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor beta. The Journal of Experimental Medicine. 1990;171:231–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fava RA, Olsen NJ, Postlethwaite AE, Broadley KN, Davidson JM, Nanney LB, et al. Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: Implications for TGF-beta-driven synovial inflammation and hyperplasia. The Journal of Experimental Medicine. 1991;173:1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Research & Therapy. 2006;8:R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Badlani N, Oshima Y, Healey R, Coutts R, Amiel D. Use of bone morphogenic protein-7 as a treatment for osteoarthritis. Clinical Orthopaedics and Related Research. 2009;467:3221–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hong JH, Lee GT, Lee JH, Kwon SJ, Park SH, Kim SJ, et al. Effect of bone morphogenetic protein-6 on macrophages. Immunology. 2009;128:e442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li C, Zheng Z. What’s the future of osteoarthritis treatment. Acta Scientific Orthopaedics. 2018;1:01–02. [Google Scholar]
- [21].Li CS, Zhang X, Peault B, Jiang J, Ting K, Soo C, et al. Accelerated chondrogenic differentiation of human perivascular stem cells with NELL-1. Tissue Engeering Part A. 2016;22:272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li C, Jiang J, Zheng Z, Lee KS, Zhou Y, Chen E, et al. Neural EGFL-Like 1 is a downstream regulator of Runt-related transcription factor 2 in chondrogenic differentiation and maturation. The American Journal of Pathology. 2017;183:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Siu RK, Zara JN, Hou Y, James AW, Kwak J, Zhang X, et al. NELL-1 promotes cartilage regeneration in an in vivo rabbit model. Tissue Engeering Part A. 2012;18:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li CS, Zheng Z, Jiang J, Jiang WL, Lee K, Berthiaume EA, et al. Neural EGFL-Like 1 regulates cartilage maturation through Runt-related transcription factor 3-mediated Indian Hedgehog signaling. The American Journal of Pathology. 2018;188:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li CS, Zheng Z, Zhang XL, Asatrian G, Chen E, Song R, et al. Nfatc1 is a functional transcriptional factor mediating NELL-1-induced runx3 upregulation in chondrocytes. Internatinal Journal of Molecular Sciences. 2018;19 (1), 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bartolome N, Szczypiorska M, Sanchez A, Sanz J, Juanola-Roura X, Gratacos J, et al. Genetic polymorphisms inside and outside the MHC improve prediction of as radiographic severity in addition to clinical variables. Rheumatology. 2012;51:1471–1478. [DOI] [PubMed] [Google Scholar]
- [27].Ho P, Bowes J, Filer CE, Bruce IN, Barton A. Investigation of Crohn’s disease and ankylosing spondylitis susceptibility loci with psoriatic arthritis. Arthritis And Rheumatism. 2008;58:S350–S350. [Google Scholar]
- [28].Polo YLBJ, Szczypiorska M, Bartolome N, Campos J, Flores-Robles BJ, Sanz J, et al. Clinical and genetic characteristics of ankylosing spondylitis patients with peripheral arthritis at disease onset. Clinical and Experimental Rheumatology. 2019;37 (2):0215–0221. [PubMed] [Google Scholar]
- [29].Klingenhoff A, Frech K, Quandt K, Werner T. Functional promoter modules can be defected by formal models independent of overall nucleoside sequence similarity. Bioinformatics. 1999;15:180–186. [DOI] [PubMed] [Google Scholar]
- [30].Desai J, Shannon ME, Johnson MD, Ruff DW, Hughes LA, Kerley MK, et al. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Human Molecular Genetics. 2006;15:1329–1341. [DOI] [PubMed] [Google Scholar]
- [31].Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. Journal of Orthopaedic Surgery and Research. 2016;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brust V, Schindler PM, Lewejohann L. Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Frontiers In Zoology. 2015;12:S1–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shen J, James AW, Zhang X, Pang S, Zara JN, Asatrian G, et al. Novel Wnt regulator NELlike molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. The American Journal of Pathology. 2016;186:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee - a study of normal and osteoarthrotic knee joints. Acta Orthopaedica Scandinavica. 1980;51:871–879. [DOI] [PubMed] [Google Scholar]
- [35].van de Loo AA, van den Berg WB. Effects of murine recombinant interleukin 1 on synovial joints in mice: Measurement of patellar cartilage metabolism and joint inflammation. Annals of the Rheumatic Diseases. 1990;49:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: Age-related differences. Annals of the Rheumatic Diseases. 1994;53:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Allen KD, Adams SB, Mata BA, Shamji MF, Gouze E, Jing LF, et al. Gait and behavior in an IL1βeta-mediated model of rat knee arthritis and effects of an IL1 antagonist. Journal of Orthopaedic Research. 2011;29:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vandeloo AAJ, Vandenberg WB. Effects of murine recombinant interleukin-1 on synovial joints in mice - measurement of patellar cartilage metabolism and joint inflammation. Annals of the Rheumatic Diseases. 1990;49:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kwak JH, Zhang YL, Park J, Chen E, Shen J, Chawan C, et al. Pharmacokinetics and osteogenic potential of Pegylated NELL-1 in vivo after systemic administration. Biomaterials. 2015;57:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lundberg C, Asberg I, Ionescu M, Reiner A, Smedegard G, Poole AR. Changes in cartilage proteoglycan aggrecan after intra-articular injection of interleukin-1 in rabbits: Studies of synovial fluid and articular cartilage. Annals of the Rheumatic Diseases. 1996;55:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Page Thomas DP, King B, Stephens T, Dingle JT. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Annals of the Rheumatic Diseases. 1991;50:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Heilborn U, Berge OG, Arborelius L, Brodin E. Spontaneous nociceptive behaviour in female mice with Freund’s complete adjuvant- and carrageenan-induced monoarthritis. Brain Research. 2007;1143:143–149. [DOI] [PubMed] [Google Scholar]
- [43].Lakes EH, Allen KD. Gait analysis methods for rodent models of arthritic disorders: Reviews and recommendations. Osteoarthritis And Cartilage. 2016;24:1837–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Amini P, Ettlin J, Opitz L, Clementi E, Malbon A, Markkanen E. An optimised protocol for isolation of RNA from small sections of laser-capture microdissected FFPE tissue amenable for next-generation sequencing. BMC Molecular Biology. 2017;18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Akkiraju H, Nohe A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. Journal of Developmental Biology. 2015;3:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kumar P, Banik S. Pharmacotherapy options in rheumatoid arthritis. Clinical Medicine Insights. Arthritis Musculoskeletal Disorders. 2013;6:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature Reviews Rheumatology. 2011;7:33–42. [DOI] [PubMed] [Google Scholar]
- [48].Berenbaum F Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis And Cartilage. 2013;21:16–21. [DOI] [PubMed] [Google Scholar]
- [49].Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Therapeutic Advances in Musculoskeletal Disease. 2013;5:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shlopov BV, Gumanovskaya ML, Hasty KA. Autocrine regulation of collagenase 3 (matrix metalloproteinase 13) during osteoarthritis. Arthritis And Rheumatism. 2000;43:195–205. [DOI] [PubMed] [Google Scholar]
- [51].Arend WP, Dayer JM. Inhibition of the production and effects of interleukin-1 and tumornecrosis-factor-alpha in rheumatoid-arthritis. Arthritis And Rheumatism. 1995;38:151–160. [DOI] [PubMed] [Google Scholar]
- [52].Pearson MJ, Herndler-Brandstetter D, Tariq MA, Nicholson TA, Philp AM, Smith HL, et al. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Scientific Reports. 2017;7:3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Goldring MB, Otero M. Inflammation in osteoarthritis. Current Opinion In Rheumatology. 2011;23:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wang MN, Sampson ER, Jin HT, Li J, Ke QH, Im HJ, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Research & Therapy. 2013;15:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. [DOI] [PubMed] [Google Scholar]
- [56].van Beuningen HM, Stoop R, Buma P, Takahashi N, van der Kraan PM, van den Berg WB. Phenotypic differences in murine chondrocyte cell lines derived from mature articular cartilage. Osteoarthritis Cartilage. 2002;10:977–986. [DOI] [PubMed] [Google Scholar]
- [57].Tan YY, Zhao G, Wang D, Wang JM, Tang JR, Ji ZL. A new strategy of minimally invasive surgery for cholecystolithiasis: Calculi removal and gallbladder preservation. Digestive Surgury. 2013;30:466–471. [DOI] [PubMed] [Google Scholar]
- [58].Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nature Protocals. 2008;3:1253–1260. [DOI] [PubMed] [Google Scholar]
- [59].Krueger F, Andrews SR. Bismark: A flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Stockwell PA, Chatterjee A, Rodger EJ, Morison IM. DMAP: Differential methylation analysis package for RRBS and WGBS data. Bioinformatics. 2014;30:1814–1822. [DOI] [PubMed] [Google Scholar]
- [61].Benjamin DJ, Berger JO, Johannesson M, Nosek BA, Wagenmakers E- J, Berk R, et al. Redefine statistical significance. We propose to change the default p-value threshold for statistical significance from 0.05 to 0.005 for claims of new discoveries. Nature Human Behaviour. 2017;1. [Google Scholar]
- [62].Aubrey J Hough J. Pathology of osteoarthritis In: Moskowitz RW, Altman RD, Hochberg MC, Buckwalter JA, Goldberg VM, editors. Osteoarthritis: Diagnosis and medical/surgical management. 4th ed. Philadelphia, PA: Wolter Kluwer/Lippincott Willams & Wilkins; 2007. p. 51–72. [Google Scholar]
- [63].Liu CL, Bai YC, Ganea D, Hart RP. Species-specific activity of rat recombinant interleukin-1-beta. Journal Of Interferon And Cytokine Research. 1995;15:985–992. [DOI] [PubMed] [Google Scholar]
- [64].Koussounadis AI, Ritchie DW, Kemp GJL, Secombes CJ. Analysis of fish IL-1 beta and derived peptide sequences indicates conserved structures with species-specific IL-1 receptor binding: Implications for pharmacological design. Current Pharmaceutical Design 2004;10:3857–3871. [DOI] [PubMed] [Google Scholar]
- [65].Yano F, Hojo H, Ohba S, Fukai A, Hosaka Y, Ikeda T, et al. A novel disease-modifying osteoarthritis drug candidate targeting Runx1. Annals of the Rheumatic Diseases. 2013;72:748–753. [DOI] [PubMed] [Google Scholar]
- [66].Aini H, Itaka K, Fujisawa A, Uchida H, Uchida S, Fukushima S, et al. Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Scientific Reports. 2016;6:18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Greenblatt MB, Ritter SY, Wright J, Tsang K, Hu D, Glimcher LH, et al. NFATc1 and NFATc 2 repress spontaneous osteoarthritis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19914–19919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Beier F NFATs are good for your cartilage! Osteoarthritis And Cartilage. 2014;22:893–895. [DOI] [PubMed] [Google Scholar]
- [69].Snelling S, Rout R, Davidson R, Clark I, Carr A, Hulley PA, et al. A gene expression study of normal and damaged cartilage in anteromedial gonarthrosis, a phenotype of osteoarthritis. Osteoarthritis Cartilage. 2014;22:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Oegema TR, Carpenter RJ, Hofmeister F, Thompson RC. The interaction of the zone of calcified cartilage and subchondral bone in osteoarthritis. Microscopy Research And Technique. 1997;37:324–332. [DOI] [PubMed] [Google Scholar]
- [71].Candela ME, Yasuhara R, Iwamoto M, Enomoto-Iwamoto M. Resident mesenchymal progenitors of articular cartilage. Matrix Biology. 2014;39:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].James AW, Shen J, Zhang X, Asatrian G, Goyal R, Kwak JH, et al. NELL-1 in the treatment of osteoporotic bone loss. Nature Communications. 2015;6:7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ranger AM, Gerstenfeld LC, Wang J, Kon T, Bae H, Gravallese EM, et al. The nuclear factor of activated t cells (NFAT) transcription factor NFATp (NFAT2) is a repressor of chondrogenesis. The Journal of Experimental Medicine. 2000;191:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wang J, Gardner BM, Lu Q, Rodova M, Woodbury BG, Yost JG, et al. Transcription factor Nfat1 deficiency causes osteoarthritis through dysfunction of adult articular chondrocytes. The Journal of Pathology. 2009;219:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yoo SA, Park BH, Yoon HJ, Lee JY, Song JH, Kim HA, et al. Calcineurin modulates the catabolic and anabolic activity of chondrocytes and participates in the progression of experimental osteoarthritis. Arthritis And Rheumatism. 2007;56:2299–2311. [DOI] [PubMed] [Google Scholar]
- [76].Miclea RL, Siebelt M, Finos L, Goeman JJ, Lowik CW, Oostdijk W, et al. Inhibition of Gsk3β in cartilage induces osteoarthritic features through activation of the canonical Wnt signaling pathway. Osteoarthritis Cartilage. 2011;19:1363–1372. [DOI] [PubMed] [Google Scholar]
- [77].Yaykasli KO, Oohashi T, Hirohata S, Hatipoglu OF, Inagawa K, Demircan K, et al. ADAMTS9 activation by interleukin 1β via NFATc1 in OUMS-27 chondrosarcoma cells and in human chondrocytes. Molecular and Cellular Biochemistry. 2009;323:69–79. [DOI] [PubMed] [Google Scholar]
- [78].Jeffries MA, Donica M, Baker LW, Stevenson ME, Annan AC, Humphrey MB, et al. Genome-wide DNA methylation study identifies significant epigenomic changes in osteoarthritic cartilage. Arthritis & Rheumatology. 2014;66:2804–2815. [DOI] [PubMed] [Google Scholar]
- [79].Wang Y, Godec J, Ben-Aissa K, Cui KR, Zhao KJ, Pucsek AB, et al. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity. 2014;40:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tokuhiro S, Yamada R, Chang XT, Suzuki A, Kochi Y, Sawada T, et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nature Genetics. 2003;35:341–348. [DOI] [PubMed] [Google Scholar]
- [81].Johnson K, Zhu ST, Tremblay MS, Payette JN, Wang JN, Bouchez LC, et al. A stem cell-based approach to cartilage repair. Science. 2012;336:717–721. [DOI] [PubMed] [Google Scholar]
- [82].Blanco FJ, Ruiz-Romero C. New targets for disease modifying osteoarthritis drugs: Chondrogenesis and Runx1. Annals of the Rheumatic Diseases. 2013;72:631–634. [DOI] [PubMed] [Google Scholar]
- [83].Li C, Zheng Z, Ha P, Chen X, Jiang W, Sun S, et al. Neurexin superfamily cell membrane receptor contactin-associated protein like-4 (Cntnap4) is involved in neural EGFL-like 1 (NELL-1)-responsive osteogenesis. Journal of Bone and Mineral Research. 2018;33:1813–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Alarcon-Riquelme ME. Role of RUNX in autoimmune diseases linking rheumatoid arthritis, psoriasis and lupus. Arthritis Research & Therapy. 2004;6:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lee YH, Bae SC, Kim JH, Seo YH, Choi SJ, Ji JD, et al. Meta-analysis of SLC22A4 and RUNX1 polymorphisms associations with rheumatoid arthritis susceptibility. Zeitschrift Fur Rheumatologie. 2015;74:351–358. [DOI] [PubMed] [Google Scholar]
- [86].Gregory MH, Capito N, Kuroki K, Stoker AM, Cook JL, Sherman SL. A review of translational animal models for knee osteoarthritis. Arthritis. 2012;2012:764621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Punzi L, Galozzi P, Luisetto R, Favero M, Ramonda R, Oliviero F, et al. Post-traumatic arthritis: Overview on pathogenic mechanisms and role of inflammation. RMD Open. 2016;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.