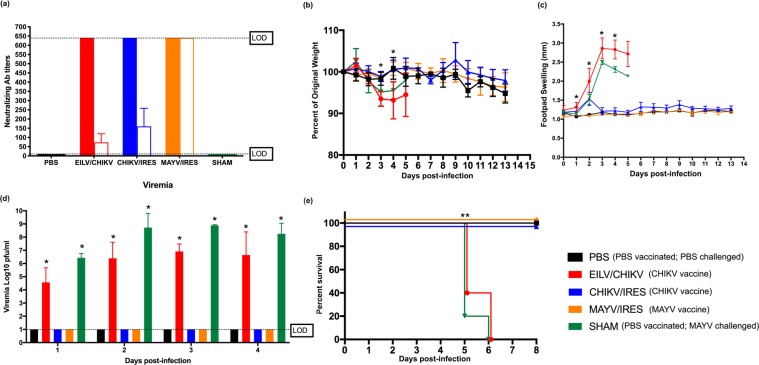
Figure 2.
CHIKV immunity provides cross-protection against MAYV infection and disease in an immunocompromised mouse model. (a) Virus-specific neutralizing antibody titers (solid bars) and MAYV cross-neutralizing antibody titers (empty bars) were determined prior to MAYV challenge. Dashed line indicates the upper and lower limits of detection (1:640 and 1:20, respectively). (b) Weight change, expressed as percent of original, and (c) footpad swelling were determined daily throughout the study. (d) Viremia was measured daily for four days post-infection and (e) survival was recorded. All plotted values in (b–d) are mean ± S.D. Data in (b–d) were analyzed using a one-way ANOVA with a Bonferroni post hoc analysis, and survival curves (e) were analyzed by Kaplan-Meier survival analysis. Footpad swelling was significantly different between the PBS control (i.e. PBS-vaccinated and PBS-challenged) and EILV/CHIKV groups on days one and two, and between the PBS control and EILV/CHIKV and sham-vaccinated groups on day three, and PBS and sham on day four. Weight change was statistically significant between the PBS control and EILV/CHIKV on days three and four. Viremia and survival were statistically significant for EILV/CHIKV and sham throughout four days post-infection. Statistically significant values are denoted by *p < 0.05.