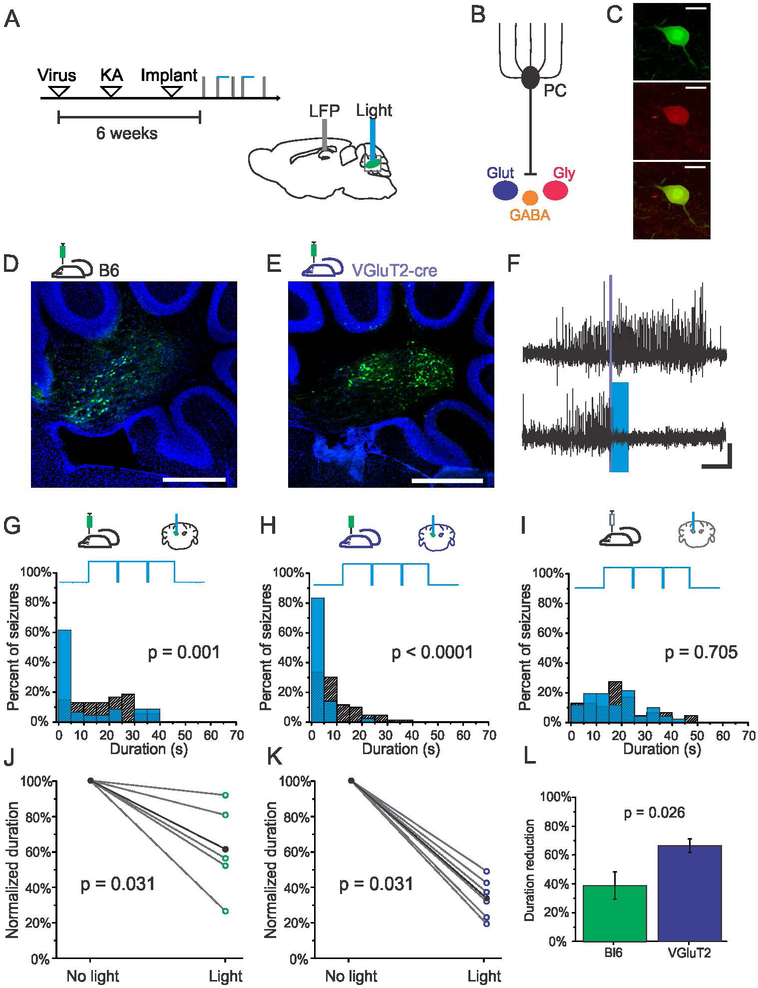
Figure 5. Selective excitation of fastigial glutamatergic neurons provides robust seizure control.
A) Schematic of experimental design and timeline. Viral approaches allowed for the targeting of nuclear neurons broadly, including nuclear glutamatergic, GABAergic, and glycinergic neurons (B), or selective targeting of glutamatergic nuclear neurons (shown in dark blue in the figure). (C) Selective expression in glutamategic neurons is achieved following injection of cre-dependent viruses in VGluT2-cre transgenic mice (Top- Green: GFP, Middle- Red: VGluT2 immunohistochemistry, Bottom- overlay, scale bar 70μm). D) GFP expression in nuclear neurons following injection in a Black-6 mouse. E) GFP expression in nuclear neurons following injection of cre-dependent virus in a VGluT2-cre mouse. Scale bars for D and E: 500μm. Green: GFP. Blue: DAPI. F) Example seizure events detected on-line (denoted by purple bar) that were either randomly selected not to receive light (top trace) or receive light (bottom trace, 3 seconds of blue (473nm) light delivery denoted by blue box). Scale bar: 5s, 0.05mV. G-H) Light delivery significantly reduces seizure duration in virally injected Black-6 (G, 44% reduction, p = 0.001, two sample Kolmogorov-Smirnov test) and VGluT2-cre (H, 81% reduction, p < 0.001, two sample Kolmogorov-Smirnov test) mice expressing channelrhodopsin. Blue bars: events receiving light intervention; hashed bars: no-light internal controls; top trace illustrates pulsed light delivery paradigm. I) No effect of light delivery in a mouse injected with AAV9-CAG-GFP control vector (p = 0.705, two sample Kolmogorov-Smirnov test). J-K) Stimulation significantly reduces the duration of hippocampal seizures across the population of channelrhodospin-expressing virally injected Black-6 (J) and VGluT2 (K) mice. Each open circle represents one animal. Black data points represent mean. L) Selective targeting of glutamatergic neurons in the fastigial nucleus produces significantly greater seizure attenuation than targeting fastigial neurons more broadly (p = 0.026, Mann-Whitney test).