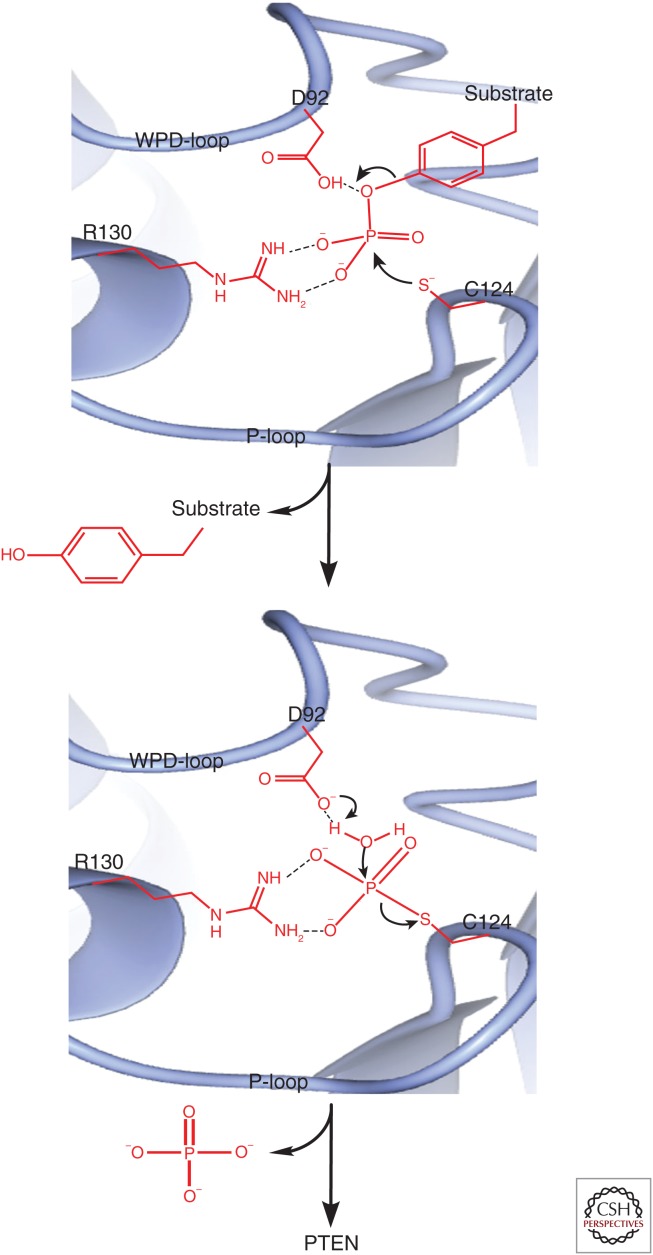
Figure 2.
The interaction of PTEN with its protein substrates at the catalytic pocket. The catalytic cysteine (C124) of the P-loop initiates a nucleophilic attack on the phosphorous atom of the phosphopeptide. This nucleophilic attack by C124 results in the breakage of the oxygen–phosphorus bond between the peptide and its phosphate group, leading to the formation of a phosphocysteine through a phosphorous–sulfur bond. This process is facilitated by aspartic acid (D92) of the WPD-loop, which donates a proton to the hydroxy leaving group on the substrate protein. The aspartic acid (D92) that donated a proton is left with a negatively charged oxygen atom, which now binds to the hydrogen on the water molecule. This allows the nucleophilic hydroxy group of the water molecule to hydrolyze the phosphorous–sulfur bond and release the free phosphate. A guanidinium group of a conserved arginine (R130) on the P-loop is needed to coordinate the position of the phosphate group during this process. (Data downloaded from the Protein Data Bank and analyzed using CCP4MG software.)