Figure 1.
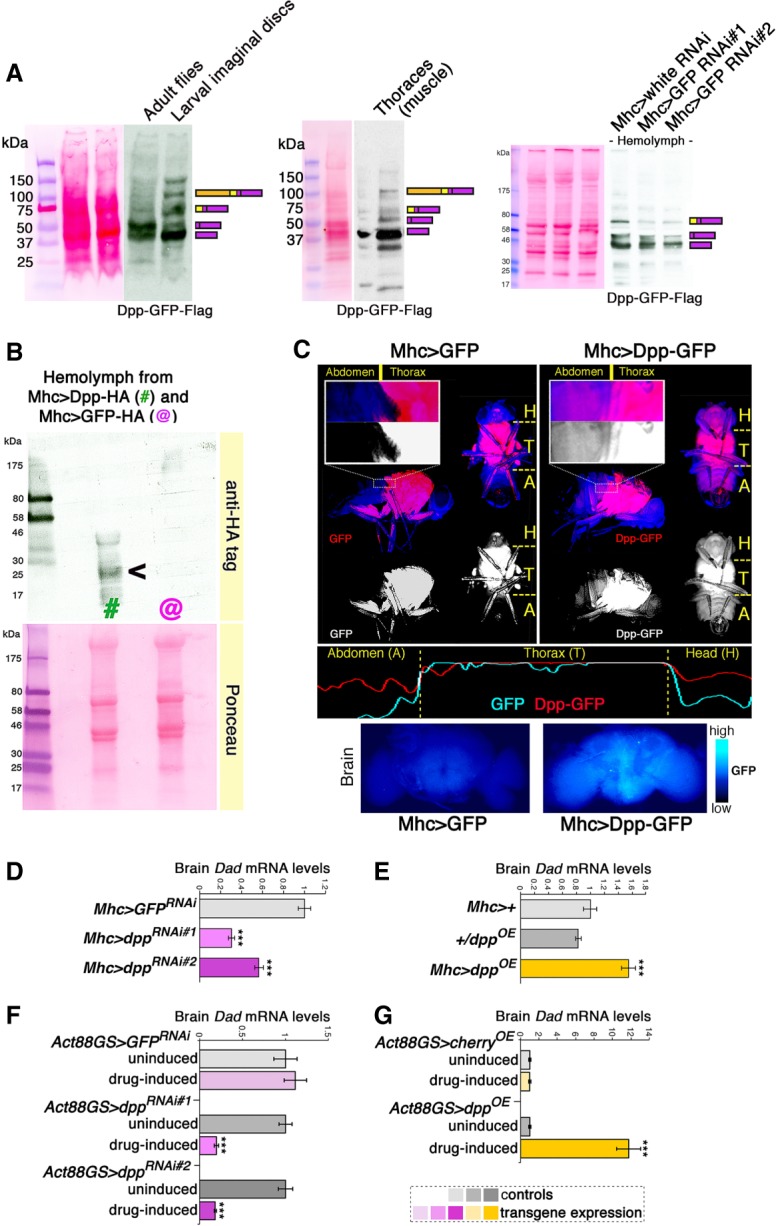
Dpp is an endocrine myokine that signals to the brain. (A) Western blot analyses indicate that endogenous Dpp-GFP-Flag is efficiently cleaved to produce mature Dpp peptides in adult flies and in skeletal muscle (thoraces). Mature Dpp-GFP-Flag is detected in the hemolymph of adult Drosophila, suggesting that it is a circulating factor. Muscle-specific GFP RNAi driven with Mhc-Gal4 leads to a ∼50% reduction in the levels of Dpp-GFP-Flag present in the hemolymph, indicating that skeletal muscle is a major source of circulating Dpp in adults. Note that the inframe fusion with GFP increases the molecular weight of Dpp-GFP-Flag, compared with the Dpp-HA shown in B. (B) Western blot analysis of hemolymph from flies expressing Dpp-HA (#; Mhc > Dpp-HA) and control GFP-HA (@; Mhc > GFP-HA) in skeletal muscle. Muscle-expressed Dpp is found in the fly circulation (hemolymph), suggesting that it is an endocrine myokine. (C) In agreement with this hypothesis, the fluorescence of muscle-produced, GFP-tagged Dpp (red) is detected in tissues of the abdomen (A) and head (H) that are distant from the production site (muscles of the fly thorax [T]). Conversely, the fluorescence of cytosolic GFP is confined to the thorax. Images of fluorescent flies and plots of fluorescence intensity are shown in C, as well as fluorescence of brains from flies with muscle-specific dpp-GFP or GFP overexpression. (D,E) Muscle-specific dpp RNAi reduces brain mRNA levels of Dad, a canonical Dpp/BMP target gene. Converse regulation is seen with muscle-specific dpp overexpression, compared with controls. (***) P < 0.001; n = 4; SEM. (F,G) Drug-induced, muscle-restricted dpp RNAi reduces brain Dad mRNA levels, whereas dpp overexpression increases it, compared with uninduced controls. (***) P < 0.001; n = 4, SEM.
