Figure 6.
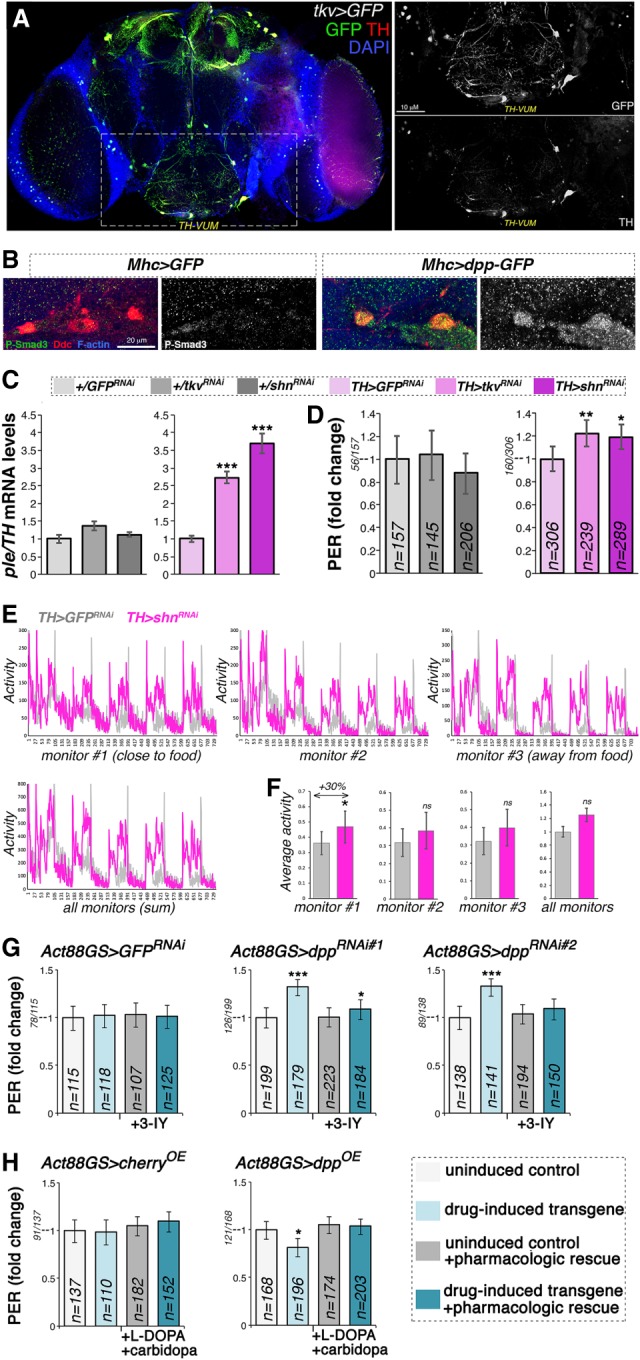
Cell-autonomous modulation of Dpp receptor signaling in dopaminergic neurons regulates ple/TH expression and the propensity to feed on sucrose. (A) Immunostaining of brains from flies that express GFP under control of the Tkv promoter (Tkv > GFP). Many neurons that express TH (red) are also positive for the Dpp receptor Tkv (green), including the TH-VUM neuron previously implicated in the PER. (B) Immunostaining of brains from flies that express GFP and dpp-GFP in skeletal muscle (Mhc > GFP vs. Mhc > dpp-GFP). Overall brain P-Smad3 immunoreactivity (indicative of BMP signaling) increases in response to muscle overexpression of dpp-GFP, and this change is particularly prominent in dopaminergic neurons. (C) RNAi for the Dpp receptor Tkv in dopaminergic neurons increases brain ple/TH levels, similar to RNAi for the transcriptional repressor Schnurri (Shn), which acts downstream from Tkv. (***) P < 0.001; n = 4; SEM. (D) RNAi for Tkv and Shn in dopaminergic neurons promotes PER. (*) P < 0.05; (**) P < 0.01; n = 145–306; 95% CI. (E) RNAi for Shn in dopaminergic neurons promotes spontaneous activity, in particular in proximity to the food (F), compared with control GFP RNAi. (*) P < 0.05; with n = 7 tubes [TH > GFPRNAi] and n = 9 tubes [TH > shnRNAi], each with 15 flies. Error bars indicate the SD. (G) The TH inhibitor 3-IY blunts PER induction by drug-induced dpp RNAi in muscle, indicating that TH is key for this adaptive response. n = 107–223; 95% CI. (H) Decrease in PER due to drug-induced, muscle-specific dpp overexpression is compensated for by the dietary administration of L-DOPA, the precursor of dopamine produced by TH. Carbidopa is coadministered to ensure preferential delivery of L-DOPA to the brain. n = 110–196; 95% CI.
