Fig. 2. Reversible oxidation of HSA upon previous persulfidation.
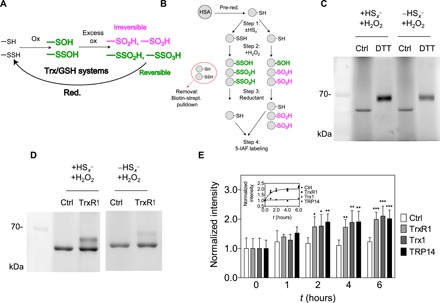
(A) Hypothesized role of the Trx or GSH system in reduction of excessively oxidized persulfide species, which become functionally reversible oxidation states (green), while nonpersulfidated Cys become irreversibly oxidized (magenta). (B) Experimental workflow for generation and detection of HSA-SSO1-3H species (for details, see Materials and Methods) (C and D) Fluorescent gels (n = 3 and 5, respectively) resulting from an experiment presented in (B) using DTT or TrxR1 as reducing agent. Excessively oxidized polysulfide species are more prone to DTT and NADPH (reduced form of nicotinamide adenine dinucleotide phosphate)/TrxR1 reduction compared to their thiol counterparts. (E) Addition of Trx1 or TRP14 does not increase the TrxR1 catalyzed reduction rate of HSA-SSO1-3H species. A representative time-resolved gel with WT TrxR1 is shown in fig. S4A (upper gel). Fluorescent signal intensities were determined densitometrically at each time point and normalized to the respective intensities at t = 0. Data points and error bars represent means ± SD of n = 5 experiments (*P < 0.05, **P < 0.01, and ***P < 0.001). The inset shows the derived kinetic curves, and the dashed lines represent a linear fit of the control (Ctrl) dataset and a single exponential least-square fit of all data points from the enzymatically reduced samples.
