Fig. 4. H2O2-dependent formation of cellular protein SxSOH in A431 cells.
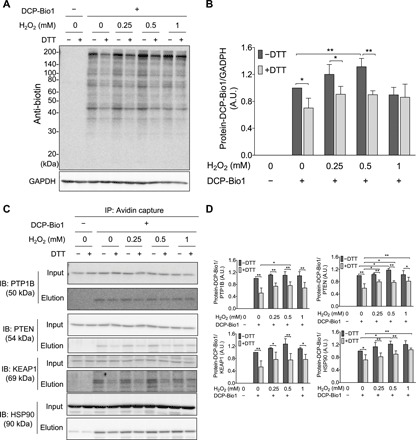
(A and B) DTT-reversible formation of oxidative modifications susceptive to dimedone labeling from whole-cell lysates. (A) A431 cells were exposed to increasing amount of H2O2 and disrupted by lysis buffer containing DCP-Bio1, a specific sulfenic acid probe containing a biotin tag. Control samples were included without the labeling agent. Cell lysates were then incubated with or without DTT, subjected to nonreducing SDS-PAGE, and immunoblotted (IB) against biotin. (B) Band intensities of the Western blot results (obtained by densitometric analyses) were plotted as a ratio of the untreated sample (─H2O2 and ─DTT). A.U., arbitrary units. (C and D) DCP-Bio1–labeled proteins were affinity-captured on avidin beads from whole-cell lysates generated as in (A), and specific proteins were identified from the eluates by Western blotting. (D) Band intensities of the membranes shown in (C). Western blots shown in (A) and (C) are representative of n = 3 independent experiments. The band intensities were assessed by densitometry using ImageJ software and normalized to (B) glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or (D) the respective protein band intensities from the input gels [whole-cell lysate before immunoprecipitation (IP)]. Each value is the mean ± SD of n = 3 independent experiments. *P < 0.05 and **P < 0.01.
