Figure 1.
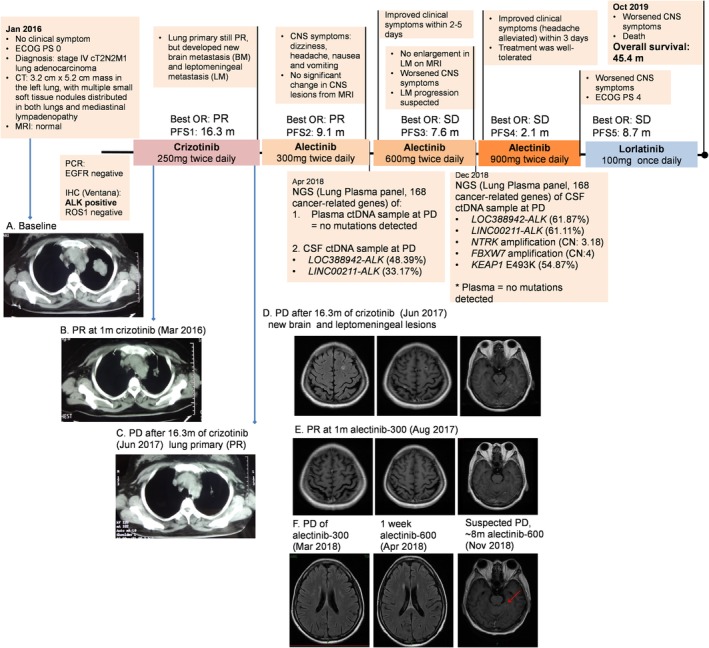
An illustrated summary of the treatment regimen received by the patient including status of clinical symptoms, investigator‐assessed objective responses (OR) based on Response Evaluation Criteria in Solid Tumors (RECIST) v.1.1, progression‐free survival (PFS) (expressed in months [m]) from each line of treatment and the mutations detected with NGS‐based profiling of CSF‐derived ctDNA and plasma‐derived ctDNA samples at indicated time points. Thoracic computed tomography (CT) at (a) baseline revealed the 3.2 cm × 5.2 cm mass in the left lung, coupled with multiple small soft tissue nodules distributed in both lungs and mediastinal lymphadenopathy. (b) At evaluation of partial response (PR) after one month of crizotinib. (c) At 16.3 months of crizotinib, CT scans of the primary lung lesions remained similar as the lesion evaluated at PR. (d) However, new metastatic lesions in the brain and meninges were revealed by cranial magnetic resonance imaging (MRI). (e) After one month of alectinib therapy at 300 mg, the size of the metastatic lesions in the skull were significantly reduced (0.4 × 0.7 cm), resulting in an evaluation of PR. (f) Subsequent MRI showing alectinib treatment course. Best OR, best overall objective response; PD, disease progression; PR, partial response; SD, stable disease.
