Abstract
The tumor microenvironment presents metabolic constraints to immunosurveiling cells. In this issue of Cancer Cell, Zhang et. al. demonstrate that CD8+ TILs reprogram under hypoxic and hypoglycemic conditions, regaining effector function by engaging fatty acid catabolism, which is promoted by fenofibrate and synergistic with immune checkpoint blockade therapy.
Metabolism regulates lymphocyte function. Distinct subsets of CD8+ T cells utilize distinct metabolic programs to engage their effector functions. When activated, CD8+ T cells employ a program hallmarked by anabolic aerobic glycolysis that is required for their growth, proliferation and production of effector molecules such as IFNγ (Pearce et al., 2013). In nutrient-replete settings, such as the tumor microenvironment (TME), the lack of glucose and other metabolites is an established mechanism of suppression of CD8+tumor-infiltrating lymphocytes(TILs). However, how TILs adapt their metabolic programs to support cancer immunosurveillance in the oxygen- and glucose-deprived TME is incompletely understood and represents a promising axis for therapeutic intervention (Buck et al., 2017).
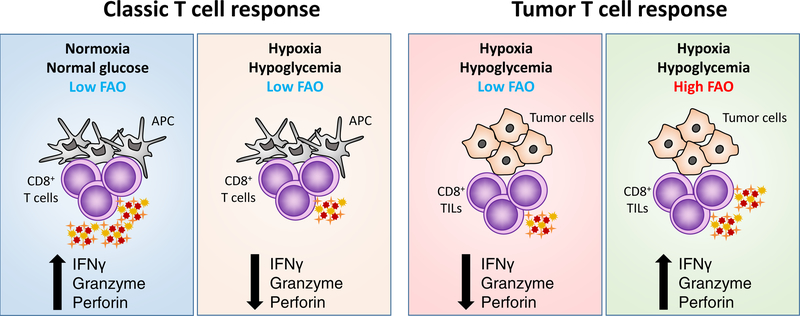
In this issue of Cancer Cell, Zhang and colleagues begin to resolve this energetic puzzle, revealing that CD8+ TILs metabolically reprogram within the TME to a catabolic state reliant on fatty acid oxidation (FAO) to sustain their effector functions (Figure 1) (Zhang et al., 2017). Zhang et. al. use an adenovirus vaccination model to elicit CD8+ responses against a suite of tumor epitopes or an irrelevant antigen, permitting the tracking of TME effects on the metabolic reprograming of both tumor specific and bystander effector CD8+ TILs. In nutrient-replete settings, such as the tumor microenvironment (TME), the lack of glucose and other metabolites is an established mechanism of suppression of CD8+tumor-infiltrating lymphocytes(TILs). However, how TILs adapt their metabolic programs to support cancer immunosurveillance in the oxygen- and glucose-deprived TME is incompletely understood and represents a promising axis for therapeutic intervention
Figure 1. Fatty acid oxidation enhances CD8+ T cell activation within the tumor microenvironment.
Effector CD8+ T cells exposed to hypoxic environments utilize glucose to generate energy and biosynthetic intermediates to maintain activation. Within the nutrient restricted solid tumor microenvironment, where both oxygen and glucose availability is low, tumor infiltrating CD8+ T cells reprogram to catabolize lipids to sustain effector function.
The authors report that although T cell activation status within the tumor is largely dictated by antigen specificity, with tumor-specific TILs exhibiting increased proliferation and expression of the checkpoint receptors PD1 and Lag3 relative to bystander TILs, the TME exerts a dominant effect on lymphocyte energetics and effector activity, with both tumor specific and bystander T cells displaying similar metabolic alterations and comparable decreases in functionality as they persist within the tumor. This decline in TIL effector quality and concurrent metabolic rewiring correlated with an increase in HIF1α expression, leading the authors to hypothesize that this important transcription factor and its role in promoting glycolysis in lieu of oxidative phosphorylation may play a key function in the reprograming process. In keeping with this hypothesis, they find that the number of TILs and their effector function is greatly enhanced upon HIF1α knockdown. Moreover, while inhibition of glycolysis in normoxic conditions led to impaired CD8+ T cell function, exposure to short-term hypoxia partially rescued dysfunction, suggesting that TILs may utilize alternate energy sources to maintain effector activity in the hypoxic TME. Of note, while PD1 expression was enhanced on antigen-specific cells utilizing glycolysis, and was impaired in the setting of hypoxia, Lag3 expression strongly correlated with HIF1α, suggesting distinct roles for antigen stimulation and metabolic status in regulating these two important checkpoint proteins.
Consistent with their in vitro observations of CD8+ T cells cultured in hypoxic, hypoglycemic conditions, Zhang and colleagues find that CD8+ TILs progressively upregulate genes associated with fatty acid catabolism and downregulate glycolytic genes, regardless of antigen specificity. The authors use metabolic flux experiments to demonstrate that, in addition to transcriptional changes, CD8+ TILs utilize less glucose and enhance mitochondrial metabolism and lipid catabolism. These changes are a specific adaptation to the TME, as cells with the same specificity from the spleen do not exhibit these alterations. To demonstrate the capacity of these metabolic adaptations to promote TIL survival and function in the TME, the authors show that treatment with fenofibrate, a PPARα agonist known to enhance fatty acid catabolism, leads to increased CD8+ T cell function both in cells cultured in hypoxic conditions and in TILs obtained from tumor bearing mice treated with the compound and transplanted to secondary recipients. To demonstrate the effect of PPARα agonism is T cell intrinsic, they transfer WT and PPARα-deficient TILs to secondary tumor bearing mice and observe major defects in PPARα-deficient TIL effector function. Highlighting the clinical potential for these observations, they demonstrate that fenofibrate treatment synergizes with anti-PD1 therapy, suggesting that metabolic intervention may be an important consideration in checkpoint blockade co-therapy development.
The findings described by Zhang et. al. add to a growing body of knowledge focused on the interplay between metabolic programs of T cells and tumor cells, with potential impact on patient therapies (Ho and Kaech, 2017). The low response rate in most tumors to immune checkpoint blockade has made the search for anti-PD1, anti-PDL1, and anti-CTLA4 co-therapies that enhance response an active area of investigation. The modulation of fatty acid catabolism in vitro and in the TME via fenofibrate affects the response to anti-PD1 treatment in the mouse model described herein highlights these pathways as potential targets. Indeed, several additional drugs which are FDA-approved for other indications exist that target PPARα, e.g., gemfibrozil.
In addition to immune checkpoint blockade, metabolic reprograming of tumor-infiltrating cells may have far-reaching implication for overcoming hurdles of chimeric antigen receptor (CAR) T cell therapies. While these strategies have been clinically successful in liquid tumors such as acute lymphoblastic leukemia, treatments targeting solid tumors have proven to be more challenging, presumably in part due to the metabolically inhospitable TME. The data presented by Zhang and colleagues bolster the argument that differences in methods of CAR-T cell generation, which elicit distinct populations of effector cells with different metabolic properties, may have important implications for clinical efficacy.
References
- Buck MD, Sowell RT, Kaech SM, and Pearce EL (2017). Metabolic Instruction of Immunity. Cell 4, 570–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P and Kaech SM (2017). Reenergizing T cell anti-tumor immunity by harnessing immunometabolic checkpoints and machineries. Curr. Opin. Immunol 46, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E, Poffenberger MC, Chang C, and Jones RG (2013). Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science 342, 1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y et al. (2017). Enhancing CD8+T cell fatty acid catabolism within metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cell, current issue. [DOI] [PMC free article] [PubMed] [Google Scholar]