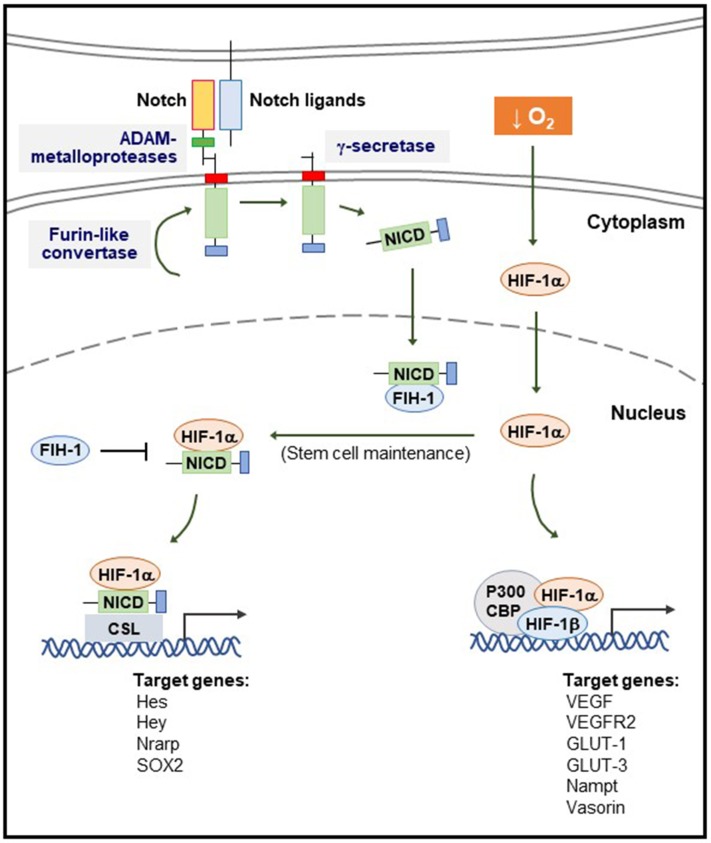
Fig. 2.
Interplay between HIF-1α and Notch during hypoxia. The inactive Notch precursor is cleaved by a Furin-like convertase and translocates into the cell membrane. The binding to a Notch ligand induces a second cleavage by the ADAM (containing a disintegrin and metalloprotease) family, resulting in the formation of a membrane-tethered Notch truncated fragment, which is further processed at two sites by a presenilin-dependent γ-secretase complex; the Notch intracellular domain (NICD), the active form of the Notch receptor, is generated. In the absence of NICD, the CSL transcription factor represses Notch target gene transcription. Following NICD activation, CSL is converted into a transcriptional activator to stimulate transcription of the same genes. Upon hypoxia and activation of Notch, HIF-1α potentiates Notch signaling through interactions with the NICD and the CSL transcription factor, leading to maintenance of the stem cell state. In the presence of factor-inhibiting HIF-1α (FIH-1), HIF-1α becomes hydroxylated at asparagine. FIH-1 blocks the interaction of the HIF-1α with the transcriptional coactivator p300-CBP (CREB binding protein). NICD can sequester FIH-1, thereby preventing hydroxylation of HIF-1α and consequently enhancing HIF-1α recruitment to hypoxia-response element sites.