Abstract
Objective
The aim of the study was to analyze the prevalence of molecular subtypes of all breast cancer patients treated at tertiary cancer centre in West India in 12 years.
Materials and Methods
A retrospective observational study carried out in Tertiary Cancer Care Centre in Western India. Electronic medical records of all breast cancer patients were retrieved from the hospital database between March 2007 to March 2019. Patient’s characteristic, histological features and molecular subtypes were collected and analyzed.
Results
A total of 2062 women fulfilled the criteria for this study and were analyzed. The median age of study population was 51 years (range 22–100 years). Among these, 1357 (65.8%) were of ≤55 years and 705 (34.2%) were over 55 years. The overall incidence of Hormonal Receptor-positive patients (either estrogen-receptor (ER) or progesterone-receptor (PR) or both) was 1162 (56.4%). The Mean tumor size was 3.8cm (range 0–18cm). The most common histology was IDC (96%). Axillary nodes were positive in 62.5%. Luminal type A was positive in 762 (37%) patients while Luminal type B was present in 157 (7.6%) patients. Basal-like subtype was observed in 537 (26%) patients while HER2 rich subtype was seen in 229 (11.1%). The incidence of Luminal A subtype increased with age. The highest observed among patients (72%) aged 70 years or more. Incidence of Basal like subtype was highest in patients less than 30 years (52%).
Conclusion
Luminal-like disease is the most common molecular subtype in India. Identification of Basal like breast cancer, a highly aggressive, biologically and clinically distinct subtype different than its non-basal variant, is important for treatment planning and target therapy.
Keywords: Retrospective observational study, molecular classification, breast cancer, immunohistochemistry, tertiary cancer centre
Introduction
Breast cancer is a global health issue among women. As per the recent GLOBOCAN 2012 data, the age-standardized incidence rate (ASR) for invasive breast cancer (females) in Asia was 29.1 per 100,000 women-years which is approximately 30% of Western population (North America has an ASR of 91.6 while Europe has an ASR of 71.1 per 100,000 women-years) (1). However, the incidence of breast cancer has increased significantly in Asian countries as compared to Western countries. Breast cancer accounts for the most frequently diagnosed cancer in Asian women. Although the incidence of breast cancer remains high in developed countries, there has been a shift in global distribution of breast cancer cases among women in South America, Africa, and Asia (2).
Pathology plays a key role in understanding complex disease such as cancer. However, in our country, there is paucity in data on key epidemiological findings (3). Are there any variances in breast cancers in India and Western literature? The answer is evidently yes. The proportion of breast cancer subtypes is different in the Indian continent.
Some of the established prognostic and predictive factors for breast cancer include tumor size, nodal involvement, histologic type, and histologic grade. Expression status by immunohistochemistry (IHC) such as estrogen receptor (ER), or progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) are key prognostic factors (4). However, these traditional classifications do not reflect the diversity of breast cancer. For example, women with HER2-negative or ER-negative tumors do not response to HER2-targeted or endocrine therapy, women with HER2-positive or ER-positive tend to show capricious responses to such targeted treatment (4). Thus, there is a need to better classify breast cancer types in order to predict outcomes in such patients.
In the past 18 years, there has been varying changes in the overall classification of breast cancer. Microarray-based gene expression profiling has helped in determining breast cancer from its histopathologic type to the molecular subtype. Today, ER-positive and ER-negative breast cancer subtypes are considered as different diseases (4). The Cancer Genome Atlas (TCGA) Network has helped established a refined subtypes of breast cancer through extensive profiling of protein levels, microRNA, and DNA (5). The molecular subtypes include “luminal A,” “luminal B,” “HER2-enriched,” and “basal-like” each of which have changed the paradigm of breast cancer treatment. The subtypes based on mRNA gene expression alone are similar to the intrinsic subtype (6). Each subtype has been associated with varying incidence, prognosis, preferential metastatic organs, response to treatment, recurrence or disease-free survival outcomes (6, 7).
Uncontrolled proliferation is a unique feature of cancer. The most common measurement of proliferation involves immunohistochemical assessment of Ki-67 antigen (8). Ki-67 has played a key role as a proliferation as it is present in all proliferating cells. Ki-67 is one of the 21 selected genes included in the Oncotype DXTM assay that has helped in predicting the extent of chemotherapy benefits and risk of recurrence among women with node negative and ER+ breast cancer. Ki-67 can have potential use in determining relative prognosis, resistance to endocrine therapy or chemotherapy, and estimation of residual risk in patients on standard therapy. It has also been used as a dynamic biomarker of treatment efficacy among patients who receive neoadjuvant therapy, specifically those who received neoadjuvant endocrine therapy (9). The St. Gallen Consensus has for years led to the development of treatment personalized towards clinical and biological subsets of breast cancer. The consensus could also be used to make informed adjuvant treatment decisions (10).
The prevalence of molecular subtypes of breast cancer have not been studied extensively in developing countries. The objective of this study is to estimate the status of different molecular subtypes of breast cancer in a tertiary cancer centre. In addition to the molecular subtypes, clinicopathological factors such as age, tumor size, and lymph node involvement have been compared.
Materials and methods
Patient population
A total of 2062 histopathologically confirmed cases of breast cancer were selected. Key factors such as age, gender, laterality, treatment-related factors, type of surgery, tumor size, histological subtype, nodal status, and molecular subtype were taken into consideration. Patient records were evaluated over a period of twelve years (March 2007 to March 2019). Age-wise distribution of molecular subtypes was also taken into consideration. Manavata Clinical Research Institute Ethics Committee approval (ACDMW-00003) was obtained prior to the commencement of the study. Informed consent from all patients were obtained.
Data collection
All records were collected from the hospital electronic medical records. The histopathological and immunohistochemical (IHC) examination was performed in accordance with the College of American Pathologists/American Society of Clinical Oncology (CAP/ASCO) guidelines.
ER and PR scoring for all cases was done using Allred scoring. ER and PR were considered positive for cases, which scored 3+ or more on Allred score. HER2 scoring was done according to the ASCO/CAP guidelines. We classified breast cancer cases in 4 subtypes based on hormonal receptor and Her2 status. This were luminal A (ER+ and/or PR+/HER2−), luminal B (ER+ and/or PR+/HER2+), HER2-enriched (ER− and PR−/HER2+) and Basal like (ER− and PR−/HER2−). Those patients who had Her2 2+ expression (Equivocal) were not included in molecular subtype analysis.
Statistical Package for the Social Sciences version 22 (IBM Corp.; Armonk, NY, USA) was used for data analysis. We used descriptive analysis to present our results.
Results
The patient’s age ranged from 22 to 100 years with a median age of 50.02 years. The 41–50 age group represented most of the patients (31.3%) followed by the 51–60 age group (27.6%), 31–40 age group (16.9%), 61–70 age group (15.4%), above 70 years (5.8%), and less than 30 years (3.1%) (Table 1). In context to gender, 99.1% (2043) comprised of females while 0.9% (19) comprised of males (Figure 1). In context to laterality, 51.2% (1056) had left-sided breast cancer, 47.4% (978) had right-sided breast cancer, followed by those with bilateral breast cancer, 17 (0.8%) (Figure 2).
Table 1.
Age-wise distribution of breast cancer patients
| Age group | Number | % |
|---|---|---|
| < 30 | 63 | 3.1 |
| 31–40 | 348 | 16.9 |
| 41–50 | 645 | 31.3 |
| 51–60 | 569 | 27.6 |
| 61–70 | 317 | 15.4 |
| >70 | 120 | 5.8 |
Figure 1.

Gender distribution of breast cancer at our centre
Figure 2.

Distribution of cases as per laterality
In context to intent of treatment, majority of our patients, i.e. 90% (1860) received radical treatment followed by 10% (202) who received palliative treatment. At our hospital, 53% (972) patients underwent modified radical mastectomy (MRM) while 47% (869) underwent breast conservation treatment (Figure 3).
Figure 3.

Distribution of cases as per intent of treatment
The mean median tumour size was 3.0 cm. The tumour size ranged between 0 to 18 cm. At our centre, patients with breast cancer present with varying histological subtypes. While 96% (1980) of the patients had invasive ductal carcinoma, 0.8% (16) had invasive lobular carcinoma and 0.5% (11) had ductal carcinoma in situ, 2.7% (55) had other histological subtypes (Figure 4). In the context of nodal involvement, 62.5% (1289) were found to have nodal involvement while 37.5% (773) had no nodal involvement (Table 2). The overall incidence of Hormonal Receptor-positive patients (either ER or PR or both) was 56.4% (1162).
Figure 4.

Distribution of cases based on histological type
Table 2.
Number of patients based on nodal involvement
| Age group | Number | % |
|---|---|---|
| Positive | 1289 | 62.5 |
| Negative | 773 | 37.5 |
Among the molecular subtypes, Luminal A was the most common one (37%) followed by basal-like (26%), HER2 rich (11.1%), and luminal B (7.6%). We also had patients with unclassified subtypes (18.3%) due to the equivocal status of HER2 receptor (Figure 5).
Figure 5.
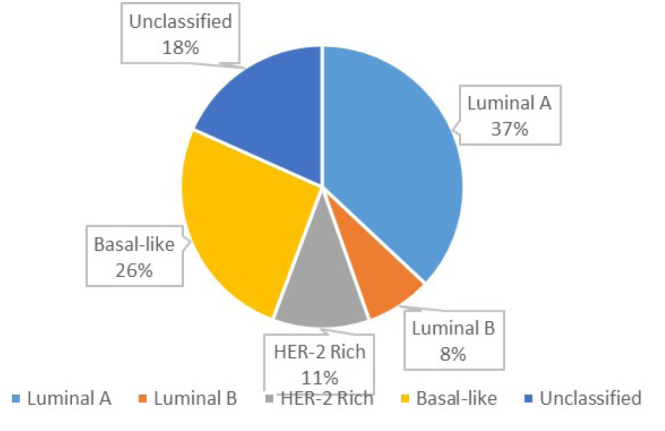
Distribution of cases as per molecular subtypes
Discussion and Conclusion
Breast cancer remains one of the leading causes of death among women globally. It is a heterogeneous and complex disease attributed to clinical, pathological, and biological factors that vary from one population to another. Identifying these prognostic factors is key for the successful management of breast cancer patients. However, molecular classification of breast cancer has emerged as a vital tool for optimal patient management. Thus, to gain insights into breast cancer and molecular subtypes among Indian women, we analyzed 2062 breast cancer patients from our hospital database. Thus, to the best of our knowledge, this paper represents one of the largest studies in India on breast cancer using a large series of patients.
The study population in our study comprised of 2062 patients ranging between 22–100 years with a mean age of 51.18 years. Our findings are similar to those reported by Mane et al. (11) In our case, 37% of patients were luminal A, 8% were luminal B, 11% were HER2 rich, and 26% were basal-like. In the case of Mane et al. (11) 43.8% were luminal A, 14.8% were luminal B, 16.1% were Basal-like, and 16.1% were HER2 rich.
The age-specific incidence rates of breast cancer vary among Western and Asian population. In Asian population, breast cancer is characterized at an early age as contrast to advancing age among Western women. The age-specific incidence decreases or plateaus after 50 years in Asian women (12–15).
In our case, luminal A (37%) was the most predominant histopathological subtype observed followed by basal-like, HER2 rich, luminal B, and other unclassified subtypes. As per international studies, the incidence of luminal A has remained predominant followed by luminal B, HER2, and basal-like (Table 3) (16–24). We also observed that the incidence of Luminal Type A subtype increases with age. The incidence rate of the luminal A subtype peaked among patients aged >70 years (72%) (Figure 6). In Our study, the incidence of luminal B cancers was much more evenly distributed, with almost similar rates among patients aged 50–59, 60–69 and >70 years respectively. In our case, basal-like histopathological subtype was found to be predominant. The rate of triple negative or basal-like subtype in our case is more or less similar to other national and international studies (16–24).
Table 3.
Incidence of various subtypes based on international studies
| Study | Luminal A | Luminal B | HER2 enriched | Basal-like | Total no of patients |
|---|---|---|---|---|---|
| British Columbia Cancer Agency [16,17] | 71% | 6% | 7% | 15% | 3348 |
| Mayo Clinic Breast Cancer study [18] | 86% | 9% | 2% | 4% | 256 |
| Vancouver General Hospital study [19] | 78% | 4% | 6% | 12% | 246 |
| University of British Columbia [20]. | 42% | 15% | 17% | 26% | 365 |
| Carolina breast cancer study [21]. | 51.4% | 15.5% | 6.6% | 26.4% | 496 |
| Dawood et al. [22]. | 65.8% | 14.3% | 4.9% | 15% | 1945 |
| Mane et al. [11] | 43.8% | 14.8% | 16.1% | 25.3% | 521 |
| Tubtimhin et al. [23] | 31.6% | 15.6% | 9.9% | 11.3% | 523 |
| Elidrissi Errahhali et al. [24] | 61.1% | 16.1% | 8.6% | 14.2% | 2260 |
| Our study | 37% | 8% | 11% | 26% | 2062 |
Figure 6.
Age-wise distribution of molecular subtypes in our study
The age-wise distribution based on molecular subtypes have been described in Figure 6. The mean tumor size in our study was 3.8 cm while 1430 (69%) of patients had a tumor size of more than 2 cm. Kumar et al. (25) from India also found similar results. They reported mean tumor size 3.4 cm and 85.8% of their cases had a tumor size more than 2 cm. However, Zhu et al. (26) reported mean size of 2.1 cm. The higher mean tumor size in our study and in India may be due to late presentation during the progression of the disease because of the existing social circumstances in this subcontinent. Another important cause may be the lack of mammographic screening program and cancer awareness.
As per the literature, HER2 rich molecular subtype is observed in about 15% to 20% of breast cancers (27). In our case, HER2 rich subtype was observed in 11.2% of patients. However, the number is less than expected as we did not include patients with equivocal (2+) Her2 receptor status. In our study, 181 (8.8%) patients had equivocal (2+) HER2 receptor expression. As per the recommendations of the American Society of Oncology and College of American Pathologist, Fluorescence in situ hybridization (FISH) could not be performed for HER2 equivocal cases. This is a major limitation as it could have helped in obtaining precise results of prevalence of molecular subtypes of this entity. In our study, the rate of HER2-enriched cancers peaked among those aged between 51–60 (Figure 6) and the distribution was most skewed toward the younger age groups.
Although we provide a comprehensive overview on the prevalence of several molecular subtypes in our institute, there are several limitations to our findings. We have not considered Ki-67, cytokeratin 5/6, and epidermal growth factor receptor-1 (EGFR-1) factors. We have not taken into consideration about menopausal status, stage, histological grade, vascular emboli status, post-and mastectomy radiation details.
Our study found that 4th & 5th decades are the most affected age groups by breast carcinoma in this region. The mean size of the tumors and axillary lymph node involvement were found to be high in this study. In conclusion, luminal A was predominant followed by basal-like, HER2 rich, and luminal B. Identification of Basal like breast cancer, a highly aggressive, biologically and clinically distinct subtype different than its non-basal variant, is important for treatment planning and target therapy.
Acknowledgements
The authors would like to thank Mr. Lyndon Fernandes and Dr. Yasam Venkata Ramesh for their medical writing assistance.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Manavata Clinical Research Institute.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - P.P., R.P., V.P., S.G., R.N.; Design - P.P., R.P.., V.P., S.G., R.P., R.N.; Supervision - P.P., S.G., R.N.; Materials - P.P., R.P., V.P., S.G., R.P., R.N.; Data Collection and/or Processing - P.P., R.P., V.P., S.G., R.P.; Analysis and/or Interpretation - P.P., R.P., S.G.; Literature Search - P.P., R.P., V.P.; Writing Manuscript - L.F., Y.V.R.; Critical Review - R.N.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Sung H, Rosenberg PS, Chen WQ, Hartman M, Lim WY, Chia KS, Wai-Kong Mang O, Chiang CJ, Kang D, Ngan RK, Tse LA, Anderson WF, Yang XR. Female breast cancer incidence among Asian and Western populations: more similar than expected. J Natl Cancer Inst. 2015;107:djv107. doi: 10.1093/jnci/djv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med. 2014;11:101–115. doi: 10.7497/j.issn.2095-3941.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangarajan B, Shet T, Wadasadawala T, Nair NS, Sairam RM, Hingmire SS, Bajpai J. Breast cancer: An overview of published Indian data. South Asian J Cancer. 2016;5:86–92. doi: 10.4103/2278-330X.187561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng CK, Schultheis AM, Bidard FC, Weigelt B, Reis-Filho JS. Breast cancer genomics from microarrays to massively parallel sequencing: paradigms and new insights. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv015. pii: djv015. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, McShane L, Paik S, Penault-Llorca F, Prudkin L, Regan M, Salter J, Sotiriou C, Smith IE, Viale G, Zujewski JA, Hayes DF. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soliman NA, Yussif SM. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol Med. 2016;13:496–504. doi: 10.20892/j.issn.2095-3941.2016.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond MEH, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mane A, Khatib KI, Deshmukh SP, Nag SM, Sane SP, Zade BP. A Comparison of Clinical Features, Pathology and Outcomes in Various Subtypes of Breast Cancer in Indian Women. J Clin Diagn Res. 2015;9:PC01–PC4. doi: 10.7860/JCDR/2015/15253.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, Nishino Y, Sobue T, Chen CJ, You SL, Mirasol-Lumague MR, Law SC, Mang O, Xiang YB, Chia KS, Rattanamongkolgul S, Chen JG, Curado MP, Autier P. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010;21:1777–1785. doi: 10.1007/s10552-010-9604-8. [DOI] [PubMed] [Google Scholar]
- 13.Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6:229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Zhang J, Wu AH, Pike MC, Deapen D. Invasive breast cancer incidence trends by detailed race/ethnicity and age. Int J Cancer. 2012;130:395–404. doi: 10.1002/ijc.26004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, Sandelin K, Derossis A, Cody H, Foulkes WD. Is Breast Cancer the Same Disease in Asian and Western Countries? World J Surg. 2010;34:2308–2324. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast Cancer Subtypes Based on ER/PR and Her2 Expression: Comparison of Clinicopathologic Features and Survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2008.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mid-Atlantic Division of the Cooperative Human Tissue Network. [Accessed December 15, 2012]. Available from: URL: http://www.cdp.nci.nih.gov/breast/prognostic_dm.html.
- 18.Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, Knowling MA, Coppin CM, Weir L, Gelmon K, Le N, Durand R, Coldman AJ, Manji M. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 19.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 20.Olson JE, Ingle JN, Ma CX, Pelleymounter LL, Schaid DJ, Pankratz VS, Vierkant RA, Fredericksen ZS, Wu Y, Couch FJ, Vachon CM, Sellers TA, Weinshilboum RM. A comprehensive examination of CYP19 variation and risk of breast cancer using two haplotype-tagging approaches. Breast Cancer Res Treat. 2007;102:237–247. doi: 10.1007/s10549-006-9324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tischkowitz M, Brunet JS, Begin LR, Huntsman DG, Cheang MC, Akslen LA, Nielsen TO, Foulkes WD. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 23.Tubtimhin S, Promthet S, Suwanrungruang K, Supaattagorn P. Molecular Subtypes and Prognostic Factors among Premenopausal and Postmenopausal Thai Women with Invasive Breast Cancer: 15 Years Follow-up Data. Asian Pac J Cancer Prev. 2018;19:3167–3174. doi: 10.31557/APJCP.2018.19.11.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elidrissi Errahhali M, Elidrissi Errahhali M, Ouarzane M, El Harroudi T, Afqir S, Bellaoui M. First report on molecular breast cancer subtypes and their clinico-pathological characteristics in Eastern Morocco: series of 2260 cases. BMC Womens Health. 2017;17:3. doi: 10.1186/s12905-016-0361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar N, Patni P, Agarwal A, Khan MA, Parashar N. Prevalence of molecular subtypes of invasive breast cancer: A retrospective study. Med J Armed Forces India. 2015;71:254–258. doi: 10.1016/j.mjafi.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Ying J, Wang F, Wang J, Yang H. Estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status in invasive breast cancer: a 3,198 cases study at National Cancer Center, China. Breast Cancer Res Treat. 2014;147:551–555. doi: 10.1007/s10549-014-3136-y. [DOI] [PubMed] [Google Scholar]
- 27.Krishnamurti U, Silverman JF. HER2 in breast cancer: a review and update. Adv Anat Pathol. 2014;21:100–107. doi: 10.1097/PAP.0000000000000015. [DOI] [PubMed] [Google Scholar]



