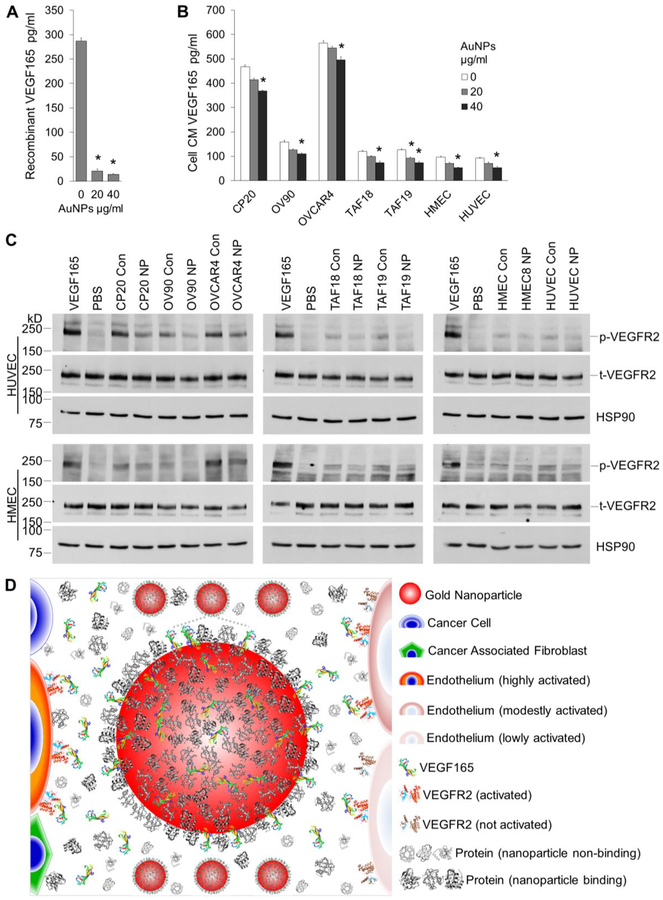
Figure 5.
VEGF quantification and signaling. (A) 1000pg/ml recombinant VEGF165 was incubated with 0, 20 or 40 μg/ml AuNPs for 16 h with agitation. VEGF165 levels in the supernatant were determined by ELISA. (B) Starved CC, CAF, or EC cells were incubated with 0, 20 or 40 μg/ml AuNP for 48 h. VEGF165 levels in CM were determined. (A, B) Experiments were performed in triplicate and repeated 3 times. *, p<0.05, compare to 0 μg/ml AuNPs. (C) Starved HUVECs or HMECs were treated with cell CM for 5 min before cell lysate preperation and protein concentration determination. Lysate with 20/40 μg HUVEC/HMEC proteins were loaded to each well in 6% SDS-PAGE gels. Equal amount of protein was loaded for phosphorylated VEGFR2 (p-VEGFR2) or total VEGFR2 (t-VEGFR2) in separate gels. HSP90 were used as loading control for phosphorylated VEGFR2. Recombinant human VEGF165 or PBS was used as positive or negative control. Experiments were repeated 3 times with similar results. Con: control CM, NP: AuNPs CM. (D) Schematic representation of the role of AuNPs in signal transduction from TME cells to ECs. TME cells secret VEGF165 and other proteins. VEGF165 binds to VEGFR2 on EC surface, phosphorylates the receptors and induces EC migration and angiogenesis. In the presence of AuNPs, VEGF165 competes with protein corona to bind to AuNPs, and less amount is available to bind to and activate VEGFR2 on ECs, leading to decreased migration and angiogenesis. Drawing not to the scale.