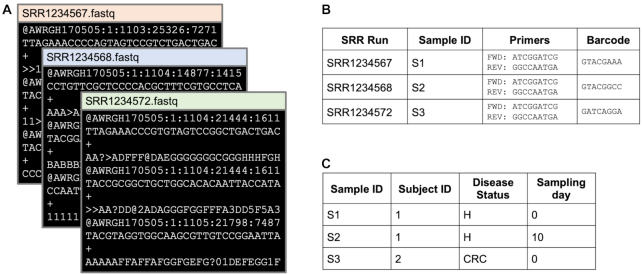
Figure 2:
Data and metadata components required for a successful re-analysis. (A) Raw sequencing data are usually labeled with an SRR Run ID or other processing ID. Raw data rarely contain information about the processing steps applied, and the research parasite must use other information to determine what processing needs to be done. (B) Technical metadata connect file names to the respective sample ID and may also have other technical information such as primer and barcode sequences. In cases in which the raw sequencing data have not been separated into sample-wise files, barcodes are required to map sequences to samples. These are often the most difficult data to find. (C) Finally, study-related metadata are required to re-analyze samples. Metadata directly related to the analysis question are always necessary (i.e., disease status), but other metadata such as subject ID, sampling day, and replicate may also be required to ensure that proper statistical comparisons are being made.