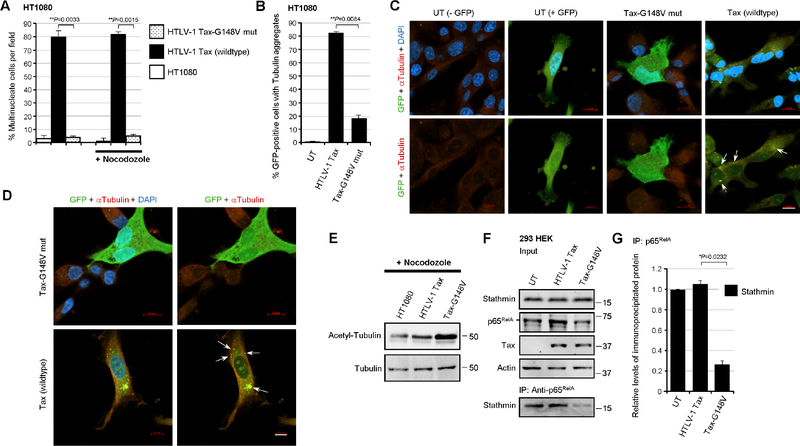
Fig. 6.
The NF-κB transactivation-defective mutant, Tax-G148V, exhibits reduced genomic instability and is impaired for p65RelA-Stathmin molecular interactions. (A) HT1080 cells were transfected with 0.5 mg of the expression constructs for wildtype HTLV-1 Tax or the Tax-G148V mutant, defective for NF-κB transactivation (Yamaoka et al., 1996), and confocal immunofluorescence-microscopy was performed to determine the relative percentages of multinucleate cells per field. Certain samples were also treated with nocodozole (400 ng/ml for 24 hrs). The samples were fixed, permeabilized, and then stained using an Anti-Tubulin primary antibody and rhodamine red-conjugated secondary antibody and DAPI. The data represent the mean ± standard deviation (error bars) from three independent experiments. (B-D) HT1080 cells were cotransfected with 0.5 mg of pcDNA3.1-GFP and the expression constructs for either wildtype HTLV-1 Tax or the Tax-G148V mutant, and the relative percentages of GFP-positive cells with cytoplasmic tubulin aggregates (arrows in C and D) were quantified in-triplicate using confocal immunofluorescence-microscopy. Representative micrographs are shown in C and D. Scale bar, 10 mm. The data in B represent the mean ± standard deviation (error bars) from three independent experiments. (E) The relative levels of acetylated tubulin in nocodozole-treated, untransfected HT1080 cells or transfected cells expressing wildtype HTLV-1 Tax or the Tax-G148V mutant were determined by SDS-PAGE and immunoblotting. Total Alpha-Tubulin protein levels are shown for comparison. (F) 293 HEK cells were transfected with the expression constructs for wildtype HTLV-1 Tax or the Tax-G148V mutant and the Stathmin protein complexed with p65RelA was co-immunoprecipitated from precleared extracts using Protein G-agarose and a monoclonal Anti-p65RelA antibody. The relative input levels of Stathmin, p65RelA, Tax, and Actin are shown in the upper panels. The precipitated Stathmin protein was detected by immunoblotting (lower panel). (G) The relative levels of immunoprecipitated Stathmin in F were quantified by densitometry analysis of the immunoblot bands. All the data in E, F and G are representative of at least three independent experiments; and the data in G represent the mean ± standard deviation (error bars).