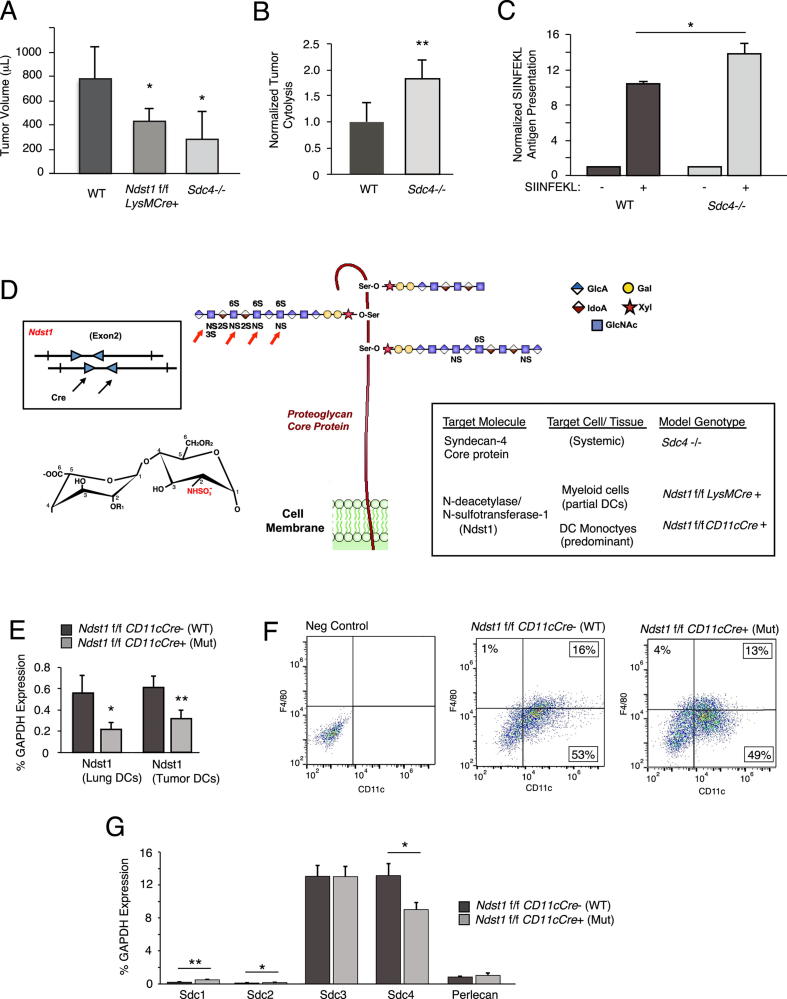
Figure 1.
Anti-tumor cellular immunity is increased in syndecan-4 deficient mice, and introduction of a genetic model to target glycan sulfation with optimum DC specificity. (A) Tumor growth of Lewis lung carcinomas (LLC) in the hindquarter of Sdc4−/− mice, Ndst1f/f LysMCre+ mutant mice bearing a myeloid-driven mutation in the major HS sulfating enzyme Ndst1, and Cre− wildtype (WT) controls was assessed at 20 days following subcutaneous inoculation of tumor cells in the hindquarter. Tumor volumes are graphed (*P = 0.05 for difference in mean with WT; **P < 0.01 for difference in mean with WT). In separate anti-tumor cytolysis studies (B), we examined a model wherein ex-vivo CD8+ T-lymphocytes harvested from the spleen of a LLC tumor-bearing mouse are combined with tumor cells purified from a whole-tumor cell digest from the same mouse, and examined for cytolysis using a dead-cell protease release assay after overnight incubation at a 20:1 T-cell/Tumor-cell effector/target ratio. Graph shows the mean degree of anti-tumor cytolysis achieved by CD8+ T cells from n = 6 Sdc4−/− mice normalized to that of n = 5 WT C57Bl/6 control mice (**P < 0.01 for difference). (C) Cultured bone marrow derived DCs from Sdc4−/− mutant and WT control mice were exposed to Ova SIINFEKL peptide, and examined for peptide presentation by flow cytometry using antibody that binds specifically to SIINFEKL in context of MHC-I. Graph shows-fold presentation of peptide-pulsed cells normalized to that of no-peptide controls run in parallel (*P = 0.02 for difference). (D) Schematic introducing strategy to optimally target the sulfation fine structure of HS glycan chains on predominantly conventional DCs. Cartoon at center shows HS polymers tethered to HSPG core protein (i.e., syndecan-4 on membrane) through O-linkage to xylose, and with wildtype HS chain oriented to left of core protein modified by clustered sulfate domains initiated by the action of Ndst: NS, 2S, 6S, and 3S sulfate groups, where Ndst mediates NS modifications on glucosamine residues (red arrows). N-sulfation modification on a chemical HS disaccharide (uronic-acid and glucosamine residues) is shown to left (where -R1 and/or -R2 may be substituted with -H and/or -SO3−. Mice with a conditional mutation in Ndst1 are crossed with CD11cCre transgenic mutants (box illustrates floxed/LoxP targets for Cre, arrows, in exon 2 of Ndst1 gene) to target HS under-sulfation to DCs, (illustrated by under-sulfated HS chains oriented to right). (E) Reduced expression of Ndst1 (qPCR, relative to GAPDH) in mutant DCs purified from lung (left histogram; from lungs of n = 3 mice per genotype; *P = 0.03 for difference) and in DCs purified from LLC tumors (right; from tumors of n = 4 mice per genotype; **P < 0.01 for difference). (F) Flow cytometry of cultured marrow-derived mutant and control DCs showing CD11c+ F4/80-DC populations and CD11c+ F4/80+ macrophage populations (13% and 16% of cells in these preparations). (G) Expression of syndecan HSPG core proteins and the secreted HSPG perlecan (as %GAPDH) in DCs from Ndst1f/f CD11cCre+ mutant and Cre− control mice (*P = 0.02 for Sdc1 expression difference, **P < 0.01 for Sdc2 difference; *P = 0.01 for Sdc4 difference).