Abstract
We present a 78-year-old male with renal cell carcinoma who developed myasthenia gravis complicated by myositis after nivolumab administration, which was verified by the presence of antibodies against the acetylcholine receptor. The initial symptom was posterior neck pain, and biochemical examination of blood showed elevated levels of hepatic enzymes and creatine phosphokinase. The level of antibody against the acetylcholine receptor increased 4.1-fold. His condition progressed rapidly resulting in respiratory failure 15 days after conservative therapy.
Abbreviations: MG, myasthenia gravis; RCC, renal cell carcinoma; AChR, acetylcholine receptor; PD-1, programmed cell death-1; irAE, immune-related adverse events; IMDC, International Metastatic RCC Database Consortium; PUL, pulmonale; PLE, pleura; ICI, immune checkpoint inhibitors; CPK, creatine phosphokinase
Highlights
-
•
The onset and progression of MG upon treating patients with the PD-1 inhibitor remains unclear.
-
•
Post-marketing surveillance data are needed to establish its true incidence.
-
•
Clinicians using PD-1 inhibitors should keep MG in mind so that appropriate treatment including steroid can be given as soon as possible to limit long-term morbidity and mortality.
Introduction
Nivolumab, a monoclonal IgG4-type antibody that recognizes programmed cell death-1 (PD-1), has been widely used for the treatment of renal cell carcinoma (RCC). Since the mechanism of action is different from that of conventional molecular-targeted drugs, there are characteristic side effects such as immune-related adverse events (irAE). Here, we report a case of nivolumab-induced myasthenia gravis (MG) complicated by myositis.
Case report
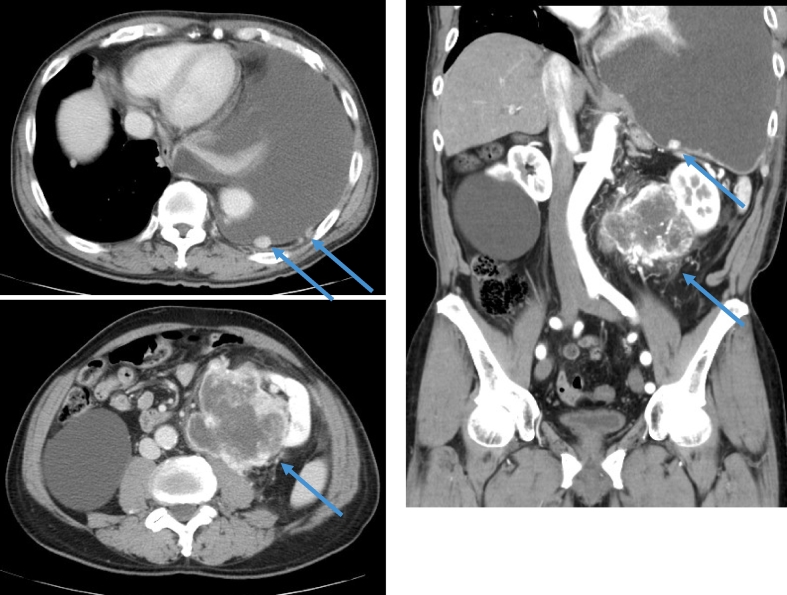
A 78-year-old man visited a referral doctor with complaints of cough and dyspnea. Computed tomography (CT) scans revealed left pleural effusion and a hypervascular tumor with a maximum diameter of 9.7 cm at the lower pole of the left kidney, which was infiltrating into the surrounding perinephric fat (Fig. 1). Blood tests showed a decreased hemoglobin level to 13.1 g/dl and an increased neutrophil fraction to 77.9% of leucocytes. RCC of this patient was classified as poor risk according to the International Metastatic RCC Database Consortium (IMDC) risk classification since it matched total 3 items, which were anemia, leucocytosis and the period of less than 1 year from the diagnosis to the start of treatment.
Fig. 1.
Computed tomography showed the tumor with a maximum diameter of 9.7 × 7.8 cm at the lower pole of the left kidney, left pleural effusion, left chest wall metastasis and left lung metastases.
We diagnosed as RCC of the left kidney (type cT4N0M1; pleura and pulmonale; IMDC poor risk) and administered a tyrosine-kinase inhibitor (TKI), pazopanib. After one month, the patient suffered from acute hepatic dysfunction and we discontinued the treatment. The time point response was a partial response (PR).
After the improvement of hepatic dysfunction, we used another TKI, axitinib. However, RCC became a progressive disease (PD) after 17 months treatment with axtinib.
The tumor metastasized to the upper portion of the right humerus that was subsequently irradiated and nivolumab was administered. After the second course of nivolumab, he complained posterior neck pain. Biochemical data of blood showed an increase in hepatic enzymes; AST and ALT levels increased to 10 and 4 times as the upper normal limit, respectively. CPK level was also elevated to 22 times as the upper normal limit. At first, we diagnosed the patient's condition as myositis and initiated steroid therapy. The posterior neck pain temporarily improved after steroid therapy. However, he soon exhibited disturbances and delirium. His symptoms gradually worsened and level of consciousness decreased. Blood gas measurements revealed a pH of 7.03 and pCO2 of 122 mm Hg, indicating CO2 narcosis. At the time that the level of antibody against acetylcholine receptor (AChR) proved to be 4.1 times as the upper normal limit, we could diagnose the patient as MG. We requested the family for informed consent to initiate intensive care with an artificial respirator. However, since the family had known that the patient would not wish any more aggressive treatments, conservative treatment was continued and the patient died 15 days later.
Discussion
In a phase III clinical trial for advanced RCC, the nivolumab group showed an advantage of overall survival about half a year longer as compared to the everolimus group.1 In contrast, nivolumab has been associated with characteristic irAE. Nivolumab-induced irAE can be observed in all organs of the body, which includes interstitial pneumonia, colitis, renal and liver dysfunction, diabetes, MG, myositis, endocrine irAE such as hypothyroidism, adrenal insufficiency and hypopgysitis, and so on. The occurrence rate of MG has been reported to be 0.12% in Japanese patients.2 Takamatsu et al. reported 17 cases of MG with high CPK levels after the use of nivolumab. Ten of these patients died and 8 out of 10 deaths were thought to be directly caused by MG.3 These 8 patients developed MG within average 16 days and died within 4 weeks after the start of nivolumab. Similarly, our patient also developed MG 2 weeks after the first nivolumab and worsened rapidly thereafter. According to a review of MG published in 2010, epidemiological data summarizing the analysis of eight studies between 1968 and 1997 showed that the mortality rate for MG is 0.06–0.89 per million people per year.4 This suggests that the prognosis of irAE associated with nivolumab-induced MG is poor when compared with non-nivolumab related MG. Thus, MG should be taken into consideration as a significant adverse event at the time of initial administration of nivolumab.
How nivolumab-mediated inhibition of PD-1 induces MG remains unclear. Recent studies have shown a link between the PD-1/L1 pathway and humoral immunity. Khan et al. emphasized the importance of PD-L1 positive regulatory B cells that suppress follicular helper T cells, which were involved in activation of memory B and plasma cells.5 When nivolumab suppressed PD-L1 positive regulatory B cells, suppression of follicular helper T cells would be released, leading to activation of memory B and plasma cells, which might be producing anti-AChR antibodies.
Conclusion
Nivolumab revolutionized the treatment for renal cell carcinoma. However.
irAE could be a serious complication. The onset and progression of MG upon treating patients with the PD-1 inhibitor remains unclear and post-marketing surveillance data are needed to establish its true incidence. Thus, clinicians using PD-1 inhibitors should keep MG in mind so that appropriate treatment including steroid can be given as soon as possible to limit long-term morbidity and mortality.
Financial conflict of interest
None.
Declaration of competing interest
None.
References
- 1.Motzer R.J., Escudier B., McDermott D.F. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki S., Ishikawa N., Konoeda F. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology. 2017;89(11):1127–1134. doi: 10.1212/WNL.0000000000004359. [DOI] [PubMed] [Google Scholar]
- 3.Takamatsu K., Nakane S., Suzuki S. Immune checkpoint inhibitors in the onset of myasthenia gravis with hyperCKemia. Ann Clin Transl Neurol. 2018 Sep 23;5(11):1421–1427. doi: 10.1002/acn3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr A.S., Cardwell C.R., McCarron P.O. A systematic review of population based epidemiological studies in Myasthenia Gravi. BMC Neurol. 2010 Jun 18;10:46. doi: 10.1186/1471-2377-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan A.R., Hams E., Floudas A., Sparwasser T., Weaver C.T., Fallon P.G. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun. 2015;6:5997. doi: 10.1038/ncomms6997. [DOI] [PubMed] [Google Scholar]