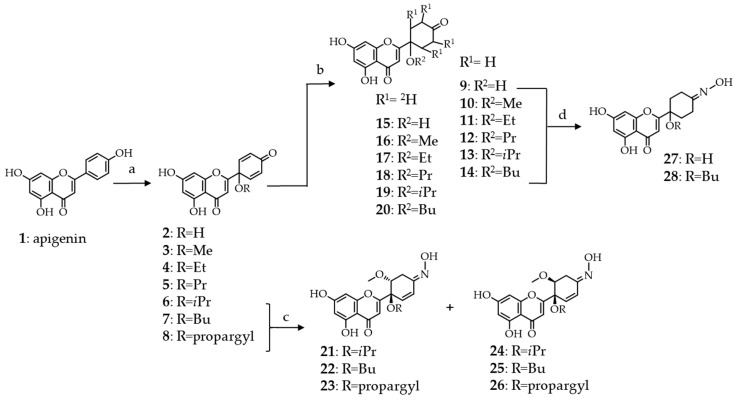
Scheme 1.
Semi-synthetic routes employed for the preparation of tetrahydro-, tetradeutero- or 4′-oxime analogs of protoapigenone and its 1′-O-alkyl ether derivatives. Oxime derivatives 21–28 were obtained as racemates, however, for simplicity only one enantiomer is shown. Reaction conditions: (a) CH3CN/ROH—9:1, PIFA (2 equiv.), 80°C, 1 h; (b) H-Cube®, 9–14: H2, 5% Pd/C or 15−20: D2, 5% Pd/BaSO4; (c) NH2OH·HCl (3 equiv.), MeOH, reflux, 24 h; and, (d) NH2OH·HCl (4 equiv.), MeOH, reflux, 3 h.