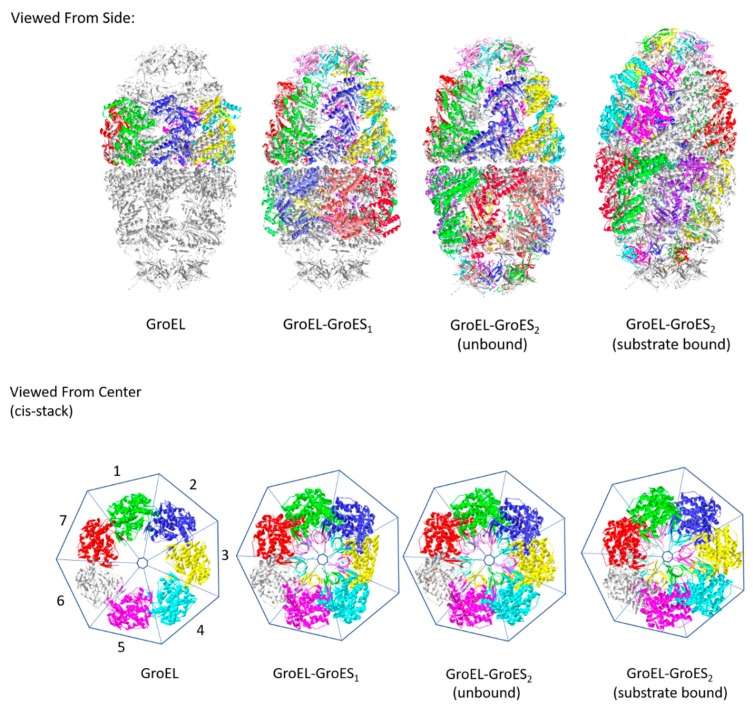
Figure 1.
Evolution of deposited Hsp60 structures in the Protein Data Bank (PDB). Colored models depict the stated structures superimposed on the full complex structure (shown in gray). The first structure was of the GroEL-stacked heptamers (PDBID: 1GRL), followed by the structures from the co-crystallized GroES and GroEL proteins (PDBID: 1AON). This GroEL–GroES1 structure is also called the “bullet” conformation. The presence of another GroES forms a “football”-shaped conformation (PDBID: 4PKO). Crystallization in the presence and absence of the polypeptide substrates show a difference in the heptamer symmetry. The full structure of a human mitochondrial chaperonin (PDBID: 4PJ1) is used as a reference for the relative positions of GroEL and GroES in the available structures. Binding to a substrate is observed to alter the heptameric symmetry of the GroEL stacks. A heptagon cage is used to approximate the relative positions of the component monomers in the different states. Changes in symmetry are observed near sectors 5–7 with GroES binding. A shift in perturbed positions is seen with the binding of a polypeptide substrate (sectors 4–6).