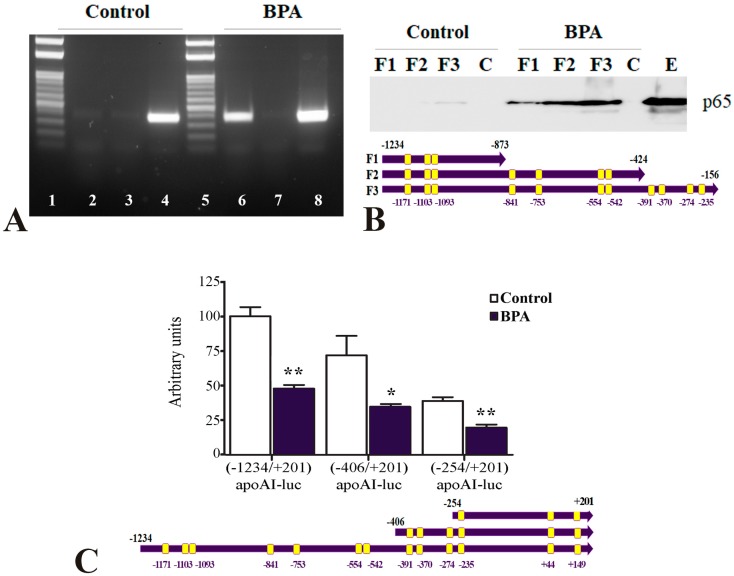
Figure 5.
NF-κB binds to apoA-I promoter in BPA-treated hepatocytes. (A) Chromatin immunoprecipitation assays showed that p65/50 proteins are recruited to the apoA-I promoter in BPA-treated HepG2 cells (lane 6). No binding of p65/50 proteins was detected when the cells were not exposed to BPA (lane 2) or when IgG antibodies were used in chromatin immunoprecipitation (lanes 3 and 7). PCR using the input prepared from BPA-treated or untreated hepatocytes as a template gave the expected product (lanes 4 and 8). The DNA ladder is represented in lanes 1 and 5. (B) DNA pull-down assays showed that p65/50 proteins bound efficiently to the three biotinylated 3′-deletion mutants of the apoA-I promoter in BPA-treated hepatocytes (BPA: F1, F2, and F3), illustrated below the graph. In untreated hepatocytes, no binding was recorded for −1234/−873 and −1234/−424 fragments of apoA-I promoter fragments (Control: F1 and F2) and a very faint band showed that p65/50 proteins weakly bound to the whole apoA-I promoter (F3, −1234/−156). No binding of p65/50 was observed to biotinylated DNA used as a negative control (Control-C or BPA-C). Western blot of p65 in the nuclear extract isolated from untreated HepG2 cells is represented in the last lane, E. (C) HepG2 cells were transfected with 5′-deletion mutants: [−1234/+201]apoA-I-luc, [−406/+201]apoAI-luc and [−254/+201]apoAI-luc (illustrated below the graph) treated with 1 µM BPA. BPA significantly decreased the activity of all the apoA-I promoter fragments tested. Statistical significance * p < 0.05, ** p < 0.01.