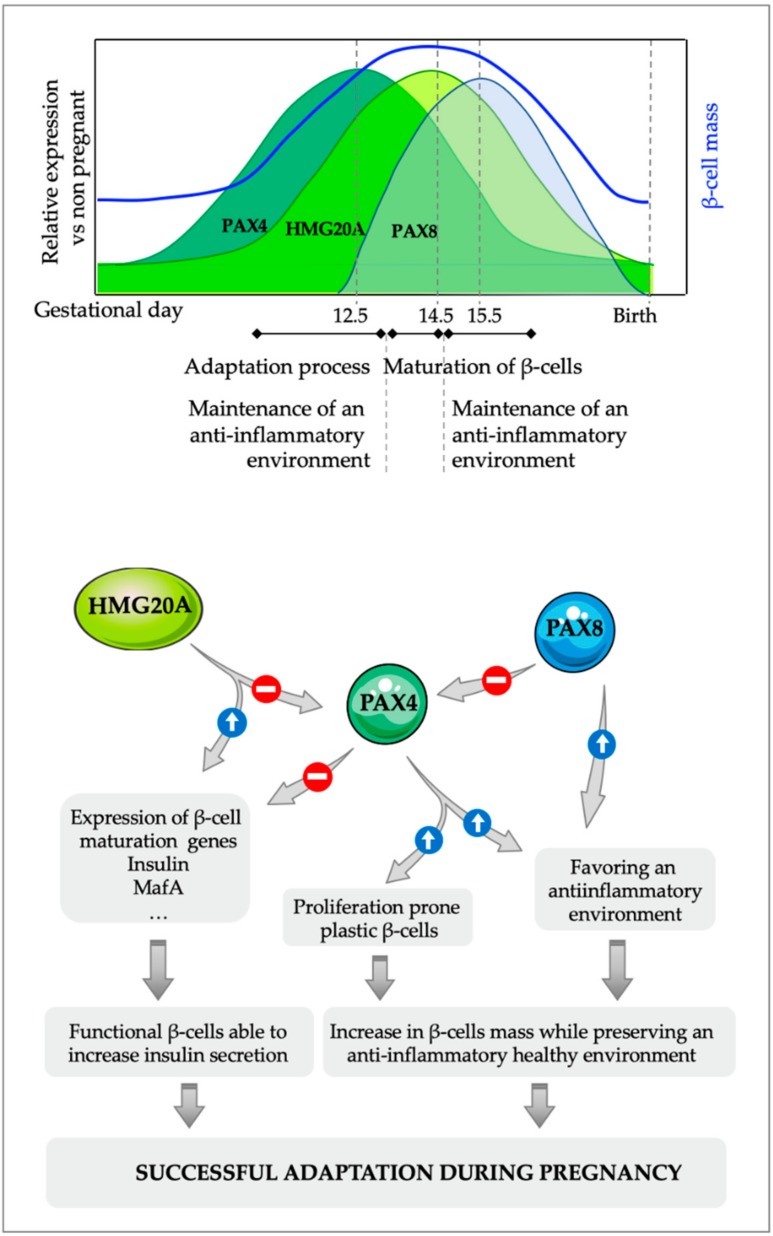
Figure 3.
Proposed molecular model for the interaction between PAX4, HMG20A and PAX8 in pancreatic β-cells during islet adaptation in response to pregnancy and inflammation. The initial increase in PAX4 during pregnancy that allows the expansion of the β-cell mass is followed by a subsequent increase in the expression of the chromatin remodeler factor HMG20A, which downregulates Pax4 expression. The decrease in PAX4 levels is necessary for the acquisition of a fully mature phenotype of these newly formed β-cells, which can then boost insulin expression/secretion to compensate for the increase in insulin demand that takes place during pregnancy. Additionally, PAX8, which is only expressed in islet during gestation, can blunt PAX4 activity through protein-protein interactions, further enhancing the inhibitory action of HMG20A on PAX4, allowing a faster maturation of the young β-cells. The combined anti-inflammatory actions of PAX4 and PAX8 will ensure a local permissive environment for the maintenance of a functional β-cell mass.