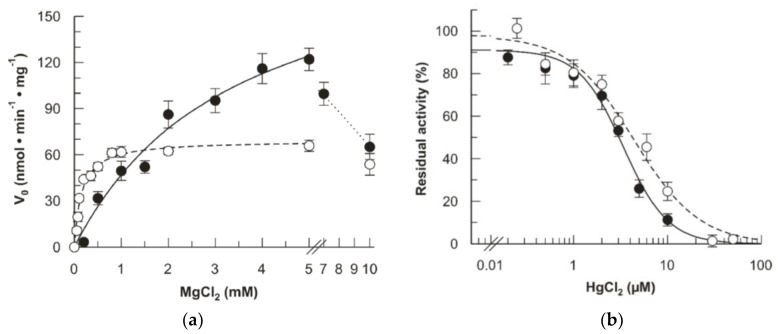
Figure 5.
Fluorimetric evidence of FAD synthesis. The FAD synthesis reaction was started by the addition of purified recombinant proteins (hFADS6 open circle or D238A-hFADS6 closed circle) and measured by the initial rate of fluorescence decrease (λ excitation = 450 nm, λ emission = 520 nm). (a) Dependence on the MgCl2 concentration. FAD synthesis rate, catalyzed by purified hFADS6 (open circle, 10 µg, 0.26 nmoL) or D238A-hFADS6 (closed circle, 2.3 µg, 0.06 nmol), was fluorimetrically measured at 37 °C in 2 mL of 50 mM Tris/HCl pH 7.5, in the presence of 100 µM ATP, 2 µM or 4 µM FMN, respectively, and of the given MgCl2 concentrations. (b) Inactivation by the mercurial reagent HgCl2. FAD synthesis rate, catalyzed by purified hFADS6 (open circle, 10 µg, 0.26 nmoL) or D238A-hFADS6 (closed circle, 3.2 µg, 0.08 nmol), was fluorimetrically measured at 37 °C in 2 mL of 50 mM Tris/HCl pH 7.5, in the presence of 2 µM or 3 µM FMN, respectively, 100 µM ATP, 5 mM MgCl2 and of the given HgCl2 concentrations. The values of V0 are reported as nmol min−1 mg−1 (a) and as percentages of the maximum rate (b) arbitrarily set to 100%. Data points are fitted according to the Michaelis–Menten equation (a) and according to the IC50 equation (b) with Grafit 3.0 software.