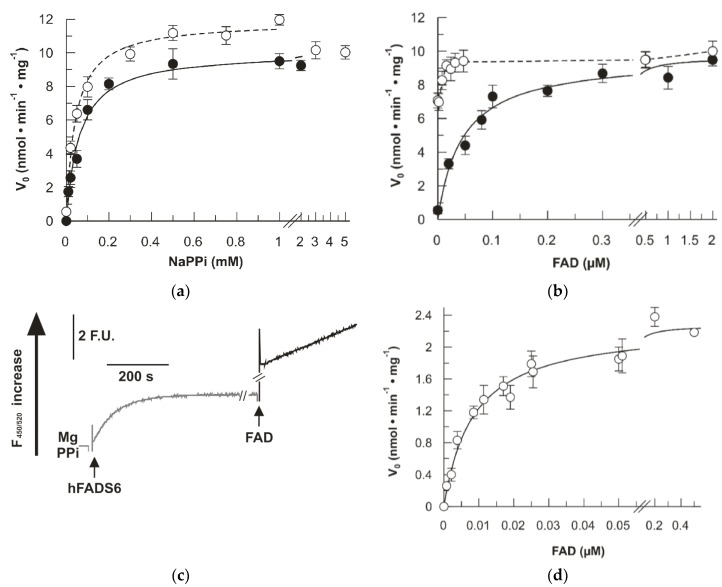
Figure 7.
Fluorimetric evidence of FAD cleavage. The FAD cleavage (i.e., pyrophosporolysis) reaction was started by the addition of purified recombinant proteins (hFADS6 open circle, or D238A-hFADS6 closed circle) and measured by the initial rate of fluorescence increase (λ excitation = 450 nm and λ emission = 520 nm). Data points are fitted according to the Michaelis–Menten equation with Grafit 3.0 software. (a) NaPPi concentration dependence. FAD cleavage (i.e., pyrophosporolysis) rate, catalyzed by purified hFADS6 (open circle, 10 µg, 0.26 nmol) or 6His-D238A-hFADS6 (closed circle, 10 µg, 0.26 nmol), was measured fluorimetrically at 37 °C in 2 mL of 50 mM Tris ⁄ HCl pH 7.5, in the presence of 5 mM MgCl2, 0.5 µM FAD, and of the given NaPPi concentrations. (b) FAD concentration dependence. FAD cleavage (i.e., pyrophosporolysis) rate, catalyzed by purified hFADS6 (open circle, 10 µg, 0.26 nmol) or D238A-hFADS6 (closed circle, 10 µg, 0.26 nmoL), was measured fluorimetrically at 37 °C in 2 mL of 50 mM Tris ⁄ HCl pH 7.5, in the presence of 5 mM MgCl2, 1 mM NaPPi, and the given FAD concentrations. (c) Exogenous FAD cleavage following endogenous FAD removal from hFADS6. The reaction catalyzed by purified hFADS6 (5 µg, 0.13 nmoL) was followed at 37 °C in 2 mL of 50 mM Tris⁄ HCl pH 7.5, in the presence of 5 mM MgCl2 and 1 mM NaPPi until the fluorescence reached a constant value corresponding to complete endogenous FAD conversion to FMN (grey line). When indicated exogenous FAD (0.5 µM) was added to calculate the rate of pyrophosporolysis (black line). (d) FAD concentration dependence of hFADS6 apo-form. FAD cleavage rate, catalyzed by apo-form of hFADS6 (5 µg, 0.13 nmoL), was measured fluorimetrically at 37 °C in 2 mL of 50 mM Tris ⁄ HCl pH 7.5, in the presence of 5 mM MgCl2, 1 mM NaPPi, and the given added FAD concentrations as described in Material and Methods (paragraph 4.6.) and graphically explained in (c).