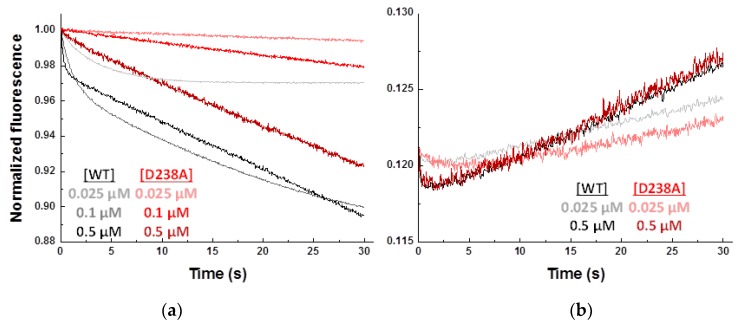
Figure 8.
Dissection of the kinetics of the substrates binding process from the catalytic reaction by stopped-flow spectrophotometry. Normalized kinetic traces for the flavin fluorescence evolution upon mixing the protein with substrates for the (a) forward (ATP + FMN) and (b) reverse (PPi + FAD) reactions. Traces for WT and D238A hFADS are shown in colored grey and red scales as a function of the flavin given concentrations. Normalized signals regarding the fluorescence of the forward reaction at maximum FMN concentration are shown. Kinetic traces were obtained at 25 °C in mixtures containing 100 nM of protein and 250 µM of either ATP (a) or PPi (b) in 50 mM HEPES/NaOH, 10 mM MgCl2, pH 7.0, 5 mM β-mercaptoethanol. All concentrations are final after mixing.