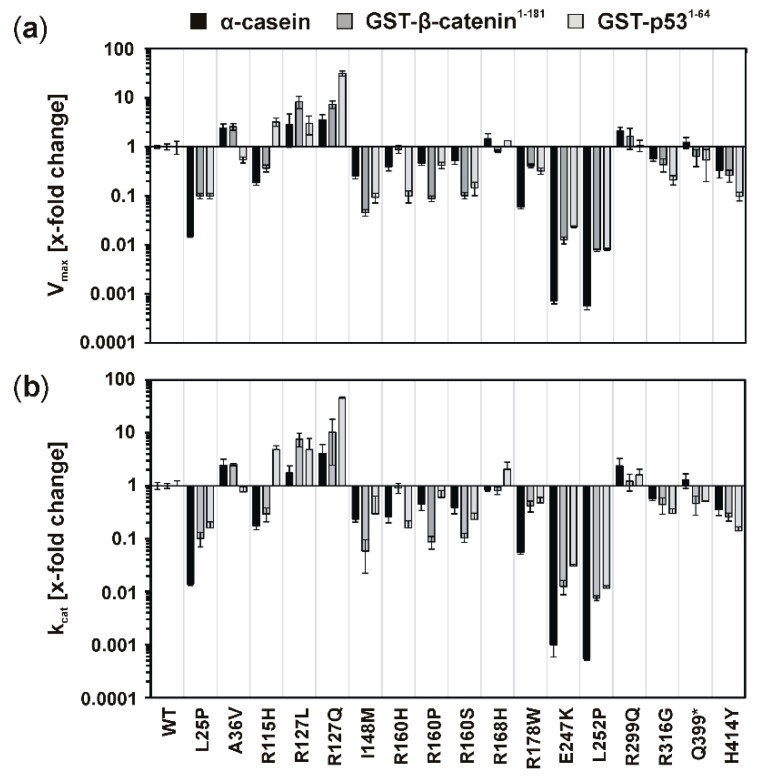
Figure 2.
Kinetic parameters Vmax and kcat of CK1δ wild type and mutants with different substrates. Michaelis–Menten kinetics have been analyzed for CK1δ wild type and mutants using either α-casein, GST-β-catenin1−181, or GST-p531−64 as substrate. The kinetic parameters Vmax (a) and kcat (b) were normalized toward the respective parameters determined for CK1δ wild type. Data is presented as mean values and standard deviation (SD) for experiments performed in triplicate. Abbreviations: A, alanine; E, glutamic acid; G, glycine; GST, glutathione S-transferase; H, histidine; I, isoleucine; K, lysine; kcat, turnover number; L, leucine; M, methionine; P, proline; Q, glutamine; R, arginine; S, serine; V, valine; Vmax, maximum enzyme reaction velocity; W, tryptophan; WT, wild type; Y, tyrosine; *, stop codon.